Describe the principle behind each of the following processes: (i) Vapour phase refining of a metal. (ii) Electrolytic refining of a metal. (iii) Recovery of silver after silver ore was leached with NaCN.
Describe the principle behind each of the following processes:
(i) Vapour phase refining of a metal.
(ii) Electrolytic refining of a metal.
(iii) Recovery of silver after silver ore was leached with NaCN.
To eliminate pollutants from the metals the way toward refining is finished. The extensively utilized technique for refining metals is electrolytic refining. Metals, for example, copper, zinc, tin, nickel, silver, gold, and so on are refined electrolytically.
The way toward removing a substance from a strong stage that interacted with fluid is known as the filtering cycle.
(i)
In this cycle, the brilliantly partitioned silver is responded to a weakened arrangement of sodium cyanide. On passing the consistent current, NaCN will go about as a fading specialist. It will oxidize silver to silver particles. This silver-particle joins with cyanide particles to give the dissolvable complex.
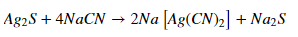
Step by step
Solved in 3 steps with 1 images









