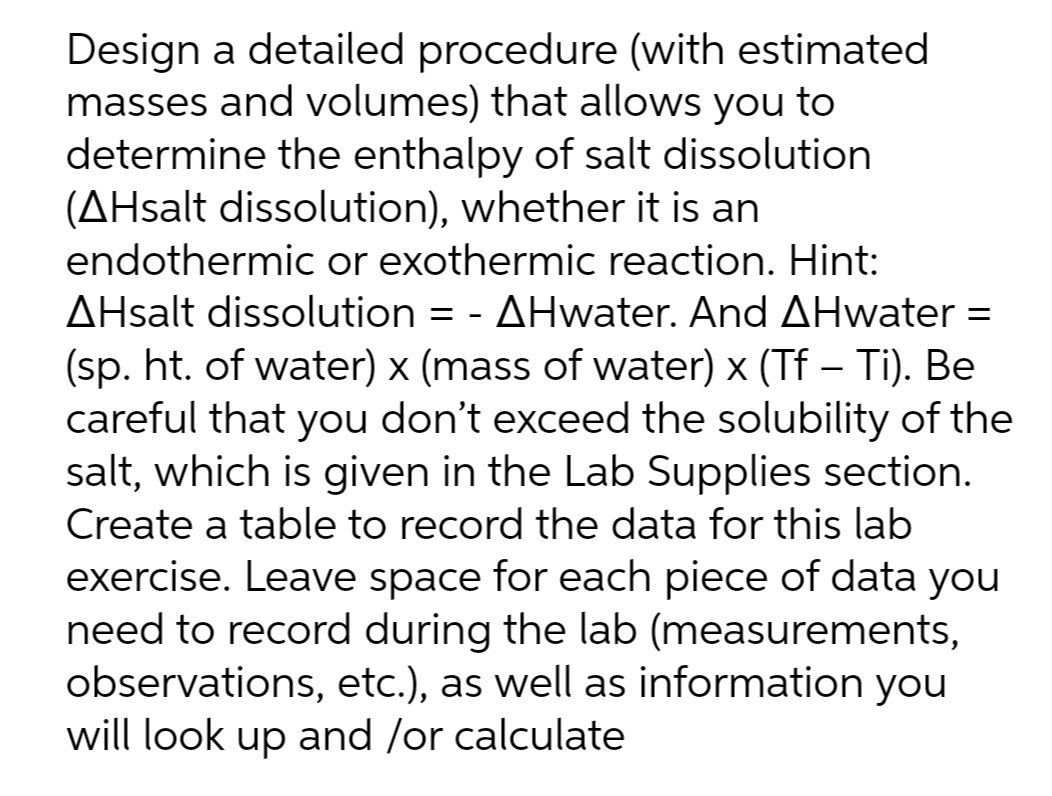
Design a detailed procedure (with estimated masses and volumes) that allows you to determine the enthalpy of salt dissolution (AHsalt dissolution), whether it is an endothermic or exothermic reaction. Hint: AHsalt dissolution = - AHwater. And AHwater = (sp. ht. of water) x (mass of water) x (Tf – Ti). Be careful that you don't exceed the solubility of the salt, which is given in the Lab Supplies section. Create a table to record the data for this lab exercise. Leave space for each piece of data you need to record during the lab (measurements, observations, etc.), as well as information you will look up and /or calculate
Design a detailed procedure (with estimated masses and volumes) that allows you to determine the enthalpy of salt dissolution (AHsalt dissolution), whether it is an endothermic or exothermic reaction. Hint: AHsalt dissolution = - AHwater. And AHwater = (sp. ht. of water) x (mass of water) x (Tf – Ti). Be careful that you don't exceed the solubility of the salt, which is given in the Lab Supplies section. Create a table to record the data for this lab exercise. Leave space for each piece of data you need to record during the lab (measurements, observations, etc.), as well as information you will look up and /or calculate
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.26QAP
Related questions
Question
4
Transcribed Image Text:Design a detailed procedure (with estimated
masses and volumes) that allows you to
determine the enthalpy of salt dissolution
(AHsalt dissolution), whether it is an
endothermic or exothermic reaction. Hint:
AHsalt dissolution = - AHwater. And AHwater =
%3D
(sp. ht. of water) x (mass of water) x (Tf – Ti). Be
careful that you don't exceed the solubility of the
salt, which is given in the Lab Supplies section.
Create a table to record the data for this lab
exercise. Leave space for each piece of data you
need to record during the lab (measurements,
observations, etc.), as well as information you
will look up and /or calculate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning