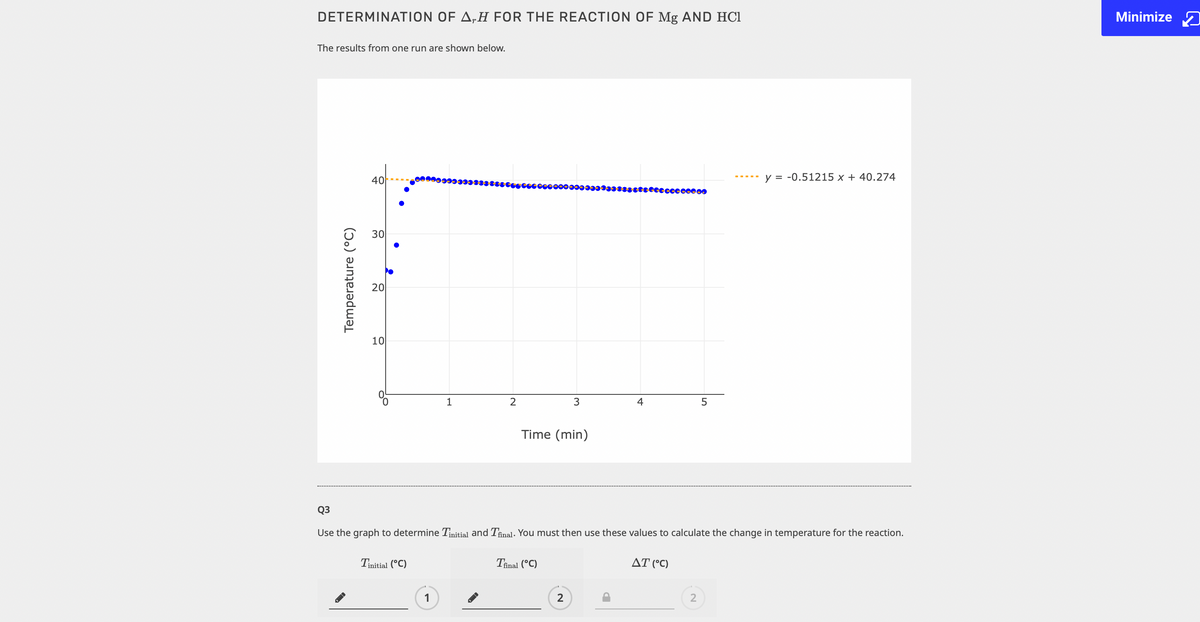
DETERMINATION OF A,H FOR THE REACTION OF Mg AND HCI The results from one run are shown below. 40 y = -0.51215 x + 40.274 30 20 10 2 Time (min) Q3 Use the graph to determine Tuitia and Thal. You must then use these values to calculate the change in temperature for the reaction. Tuna ("C) Thnai ("C) AT ("C) Temperature (°C)
DETERMINATION OF A,H FOR THE REACTION OF Mg AND HCI The results from one run are shown below. 40 y = -0.51215 x + 40.274 30 20 10 2 Time (min) Q3 Use the graph to determine Tuitia and Thal. You must then use these values to calculate the change in temperature for the reaction. Tuna ("C) Thnai ("C) AT ("C) Temperature (°C)
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter21: Rates Of Chemical Reactions, Ii. A Clock Reaction
Section: Chapter Questions
Problem 2ASA
Related questions
Question
Transcribed Image Text:DETERMINATION OF A,H FOR THE REACTION OF Mg AND HCl
Minimize D
The results from one run are shown below.
40
00003333333t1&C66C0000000000333333113ttteeccc000000
y = -0.51215 x + 40.274
20
10
4
5
Time (min)
Q3
Use the graph to determine Tinitial and Tfinal. You must then use these values to calculate the change in temperature for the reaction.
Tinitial (°C)
Tinal (°C)
AT (°C)
1
2
Temperature (°C)
30
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole
