DETERMINATION of HEAT of SOLUTION by SOLUBILITY METHOD Experimental data are given as follows, related to the temperature dependence of the solubility of ammonium nitrate in water under constant pressure. Using the equation that obtained by taking the integral of Van't Hoff equation, calculate the heat of solution, AHs by analytically 20 40 60 T(°C) S (g/100cm') 150 297 410
DETERMINATION of HEAT of SOLUTION by SOLUBILITY METHOD Experimental data are given as follows, related to the temperature dependence of the solubility of ammonium nitrate in water under constant pressure. Using the equation that obtained by taking the integral of Van't Hoff equation, calculate the heat of solution, AHs by analytically 20 40 60 T(°C) S (g/100cm') 150 297 410
Sustainable Energy
2nd Edition
ISBN:9781337551663
Author:DUNLAP, Richard A.
Publisher:DUNLAP, Richard A.
Chapter14: Ocean Thermal Energy Conversion And Ocean Salinity Gradient Energy
Section: Chapter Questions
Problem 8P
Related questions
Question
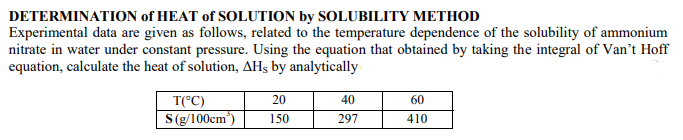
Transcribed Image Text:DETERMINATION of HEAT of SOLUTION by SOLUBILITY METHOD
Experimental data are given as follows, related to the temperature dependence of the solubility of ammonium
nitrate in water under constant pressure. Using the equation that obtained by taking the integral of Van't Hoff
equation, calculate the heat of solution, AHs by analytically
T(°C)
20
40
60
S(g/100cm)
150
297
410
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, civil-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you


Materials Science And Engineering Properties
Civil Engineering
ISBN:
9781111988609
Author:
Charles Gilmore
Publisher:
Cengage Learning


Materials Science And Engineering Properties
Civil Engineering
ISBN:
9781111988609
Author:
Charles Gilmore
Publisher:
Cengage Learning