Determination of the Universal Gas Constant Postlab 1. An evaluation of Rwas performed using the procedures outlined in this exercise, The barometric pressure was 735 torr and the temperature was 21.5 "C. The volume of H2(g) collected was 32.7 mL. The calculated value of Rwas 0.0817 L-atm/mol-K. a. How many grams of Mg metal were used in this determination? 0.0316 b. If the correction for the vapor pressure of water had not been performed, what would be the calculated value of R? 70.361 Incorrect c. If the syringe volume had been incorrectly read, resulting in a calculated H, volume of 30.6 ml, what would be the percent error in the calculated value of Rwith respect to the originally calculated value (0.0817 L-atm/mol-K)? 6.4 %
Determination of the Universal Gas Constant Postlab 1. An evaluation of Rwas performed using the procedures outlined in this exercise, The barometric pressure was 735 torr and the temperature was 21.5 "C. The volume of H2(g) collected was 32.7 mL. The calculated value of Rwas 0.0817 L-atm/mol-K. a. How many grams of Mg metal were used in this determination? 0.0316 b. If the correction for the vapor pressure of water had not been performed, what would be the calculated value of R? 70.361 Incorrect c. If the syringe volume had been incorrectly read, resulting in a calculated H, volume of 30.6 ml, what would be the percent error in the calculated value of Rwith respect to the originally calculated value (0.0817 L-atm/mol-K)? 6.4 %
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.127QP: A 1.000-g sample of an unknown gas at 0C gives the following data: P(atm) V (L) 0.2500 3.1908 0.5000...
Related questions
Question
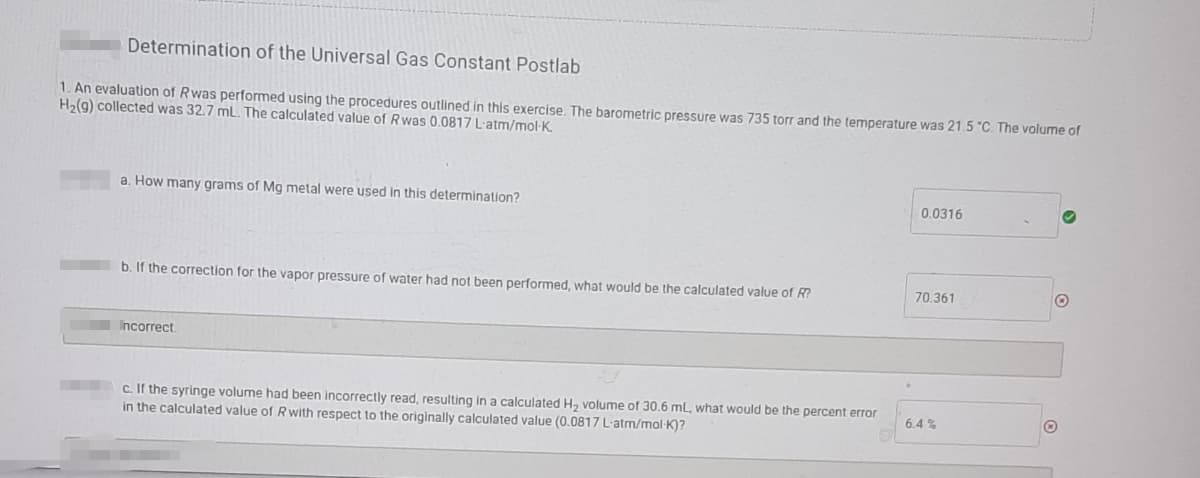
Transcribed Image Text:Determination of the Universal Gas Constant Postlab
1. An evaluation of Rwas performed using the procedures outlined in this exercise, The barometric pressure was 735 torr and the temperature was 21.5 "C. The volume of
H2(9) collected was 32.7 mL. The calculated value of Rwas 0.0817 L'atm/mol-K.
a. How many grams of Mg metal were used in this determination?
0.0316
70.361
b. If the correction for the vapor pressure of water had not been performed, what would be the calculated value of R?
Incorrect
c. If the syringe volume had been incorrectly read, resulting in a calculated H, volume of 30.6 mL, what would be the percent error
in the calculated value of Rwith respect to the originally calculated value (0.0817 L-atm/mol·K)?
6.4 %
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning