Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter12: Unsaturated Hydrocarbons
Section: Chapter Questions
Problem 12.67E
Related questions
Question
100%
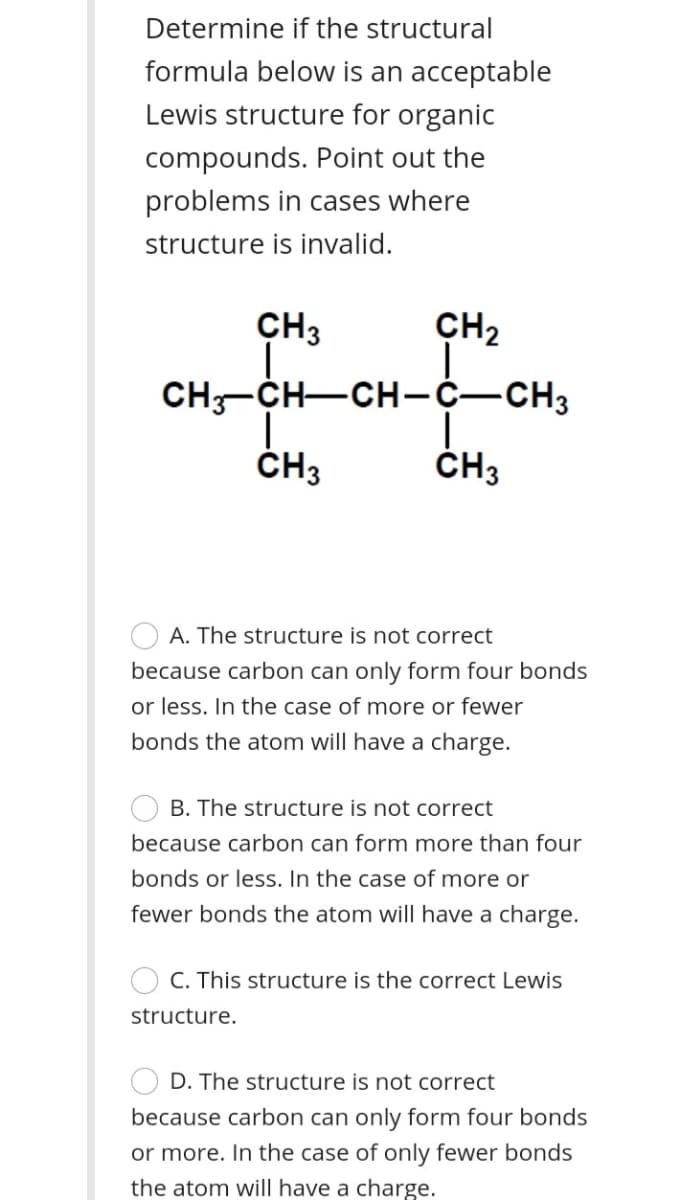
Transcribed Image Text:Determine if the structural
formula below is an acceptable
Lewis structure for organic
compounds. Point out the
problems in cases where
structure is invalid.
CH3
CH2
CH-CH-CH-C-CH3
ČH3
ČH3
A. The structure is not correct
because carbon can only form four bonds
or less. In the case of more or fewer
bonds the atom will have a charge.
B. The structure is not correct
because carbon can form more than four
bonds or less. In the case of more or
fewer bonds the atom will have a charge.
C. This structure is the correct Lewis
structure.
D. The structure is not correct
because carbon can only form four bonds
or more. In the case of only fewer bonds
the atom will have a charge.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning