Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 7P
Related questions
Question
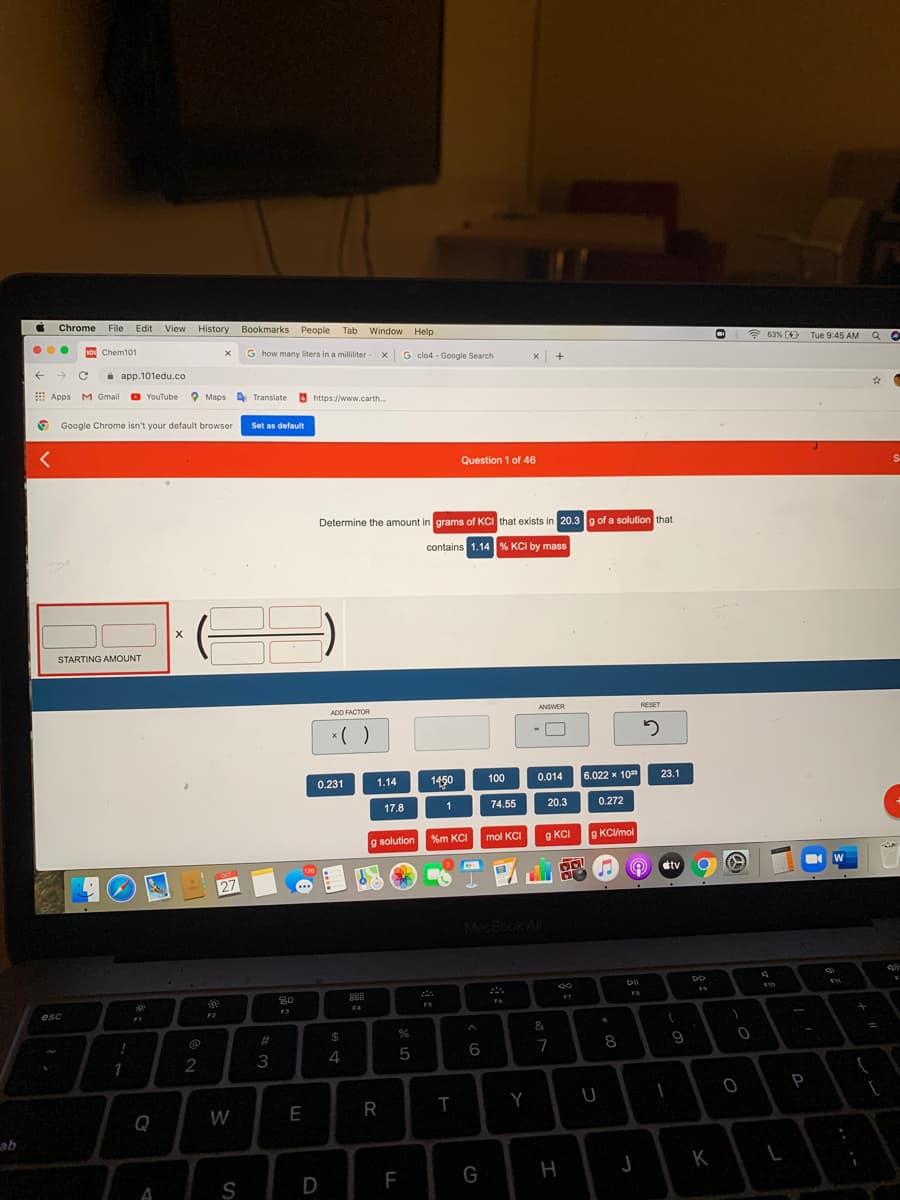
Transcribed Image Text:i Chrome
File
Edit View History Bookmarks People Tab Window Help
1o Chem101
G how many liters in a milliliter X
* 63% (4) Tue 9:45 AM Q O
G clo4 - Google Search
i app.101edu.co
E Apps M Gmail O
YouTube 9 Maps
A Translate
https://www.carth.
O Google Chrome isn't your default browser
Set as default
Question 1 of 46
Determine the amount in grams of KCI that exists in 20.3g of a solution that
contains 1.14 % KCI by mass
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
0.231
1450
100
0.014
6.022 x 10
1.14
23.1
17.8
74.55
20.3
0.272
%m KCI
g KCI
g KC/mol
g solution
mol KCI
oct
126
etv
27
MacBook Air
30
888
F7
F6
FS
esc
F2
F3
F4
&
@
#3
2$
4
5
6
7
8
2
3
P
Y
Q
W
E
K
S
D
F
G
A
I
R
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning