Q: Protons passing through ATP synthase make it rotate Kinetic energy of protons is used to power phosp...
A: According to the question, we need to determine the wrong statement. We know that: 1. Protons flow ...
Q: Write the five balanced reactions of the copper cycle, including phase symbols. Number them 1 throug...
A: Copper cycle involves five reactions.In the first reaction, copper metal is oxidized by nitric acid ...
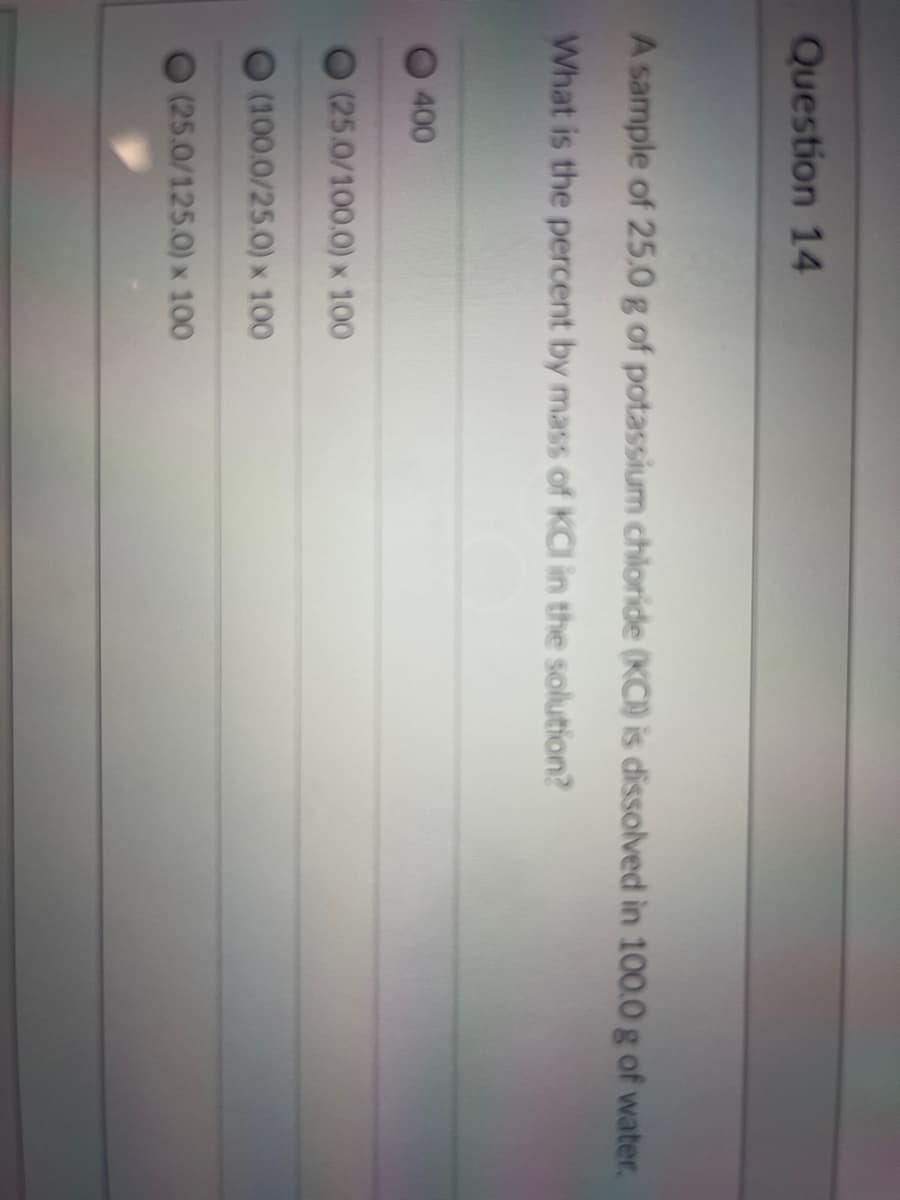
Q: how would you prepare 9 liters of a 50% dye solution beginning with a 60% stock dye solution?
A: Given :- Initial concentration or concentration of stock solution = 60% Final concentration or con...
Q: What is the pH of a solution with hydroxide ion concentration
A: pH = - log[H+] At 25° C [H+] [OH-] = 10-14 Given that [OH-] = 0.0076 M [H+] (0.0076 M) = 10-1...
Q: Consider the following reaction at equilibrium. What effect will removing NO2 have on the system? SO...
A:
Q: What is the pOH of a solution that has [OH^-] = 2.7E-4 M
A: pOH can be expressed as -log[OH-]. It is used to represent basicity of a solution. If the pOH value ...
Q: Activity 2: Complete the table below. Lewis Bond Molecular Polarity of Molecule Structure EN Polarit...
A:
Q: What are the Miller indices of the slip directions: (a) on the (111) plane in an FCC unit cell? (b) ...
A: To determine the Miller indices of slip directions for the (111) plane in FCC unit cell and (011) in...
Q: Draw the structure of the following compounds. Use line-angle structural formula. 1-bromo-4-ethyl-5-...
A:
Q: It is only possible to have pi delocalization if there are no unhybridized p-orbital in a carbon ato...
A: Answer: False Short summery: Delocalization of pi electron only possible if molecule has unhybrid...
Q: Describe the preparation of the following reagents. 3. 375 mL of 40 ppt Cl" from 0.01 M PbCl2
A: A numerical problem based on concentration terms that is to be accomplished.
Q: Calculate the "Third Law" entropy of CCl4 gas at 200 °C from the following data for CCI4- o S'298(li...
A:
Q: amount of ammonia s
A: In this question we have to explain what is the amount of ammonia depend on in the process of adjust...
Q: A student heats a 5.0 g sample of an unknown metal to a temperature of 207∘C, and then drops the sam...
A:
Q: A mixture initially contains A, B, and C in the following concentrations: [A] = 0.400 M , [B] = 1.40...
A: We have to predict the Kc value.
Q: volume
A:
Q: What are the rankings of stability for regular (primary, secondary, tertiary), allylic (1, 2, 3), an...
A: Radicals are electron deficient species. Greater the number of electron donating group present, high...
Q: Several brands of antacids use aluminum hydroxide to react with stomach acid (which is primarily hyd...
A:
Q: Describe the preparation of the following reagents. 3. 375 mL of 40 ppt Cl" from 0.01 M P6CI2
A: Solution -
Q: 2. Propose a multistep synthesis for the following synthetic transformation: OH 3. How would you act...
A:
Q: the concentration in
A:
Q: A solution containing an unknown chemical has a density of 5.71 kg/L, what is the mass (in pounds) o...
A:
Q: Part Á The activation energy of an uncatalyzed reaction is 88 kJ/mol. The addition of a catalyst low...
A:
Q: Consider the equilibrium system described by the chemical reaction below, which has a value of Kp eq...
A: This question is from chapter Chemical Equilibrium. We are given a chemical reaction whose Kp is giv...
Q: Which of the following structures is ethyl (S)-2-formylbutanoate? OH B C D
A: Note : Since you have posted multiple questions, we are entitled to answer the first only. Please re...
Q: Calculate the cell potential of the cell: Use Nernst Equation and Debye Huckel Law Pb(s)PbBr2(s) | C...
A: Nernst equation is the equation for determining the cell potential for a given cell for a particular...
Q: Calculate the approximate boiling point (in °C) of a solution of 285 g of magnesium chloride in 2.0 ...
A: Given, Mass of magnesium chloride (MgCl2) = 285 g Mass of water solvent = 2.0 Kg Boiling point eleva...
Q: Calculate the activity of La3+ in a saturated solution of La(IO3)3. Ksp = 1.0 x 10-11 1.2 x 10-3 ...
A:
Q: Assigned concentration for stock solution (M) Amount of "solute" used (mg) Molar mass "solute" used ...
A: Mass of active ingredient of the ascorbic acid tablet = 1000 mg Amount of solute used = 1000 mg = 1....
Q: Explain the reasoning for the baking soda and vingear balloon experiment. Fill in the blank. If baki...
A: When baking soda and vinegar are mixed together then the formation of carbon dioxide gas take place ...
Q: Q< K the reaction goes to the right (toward products) the reaction goes to the left (toward reactant...
A:
Q: on the f onditions needed to form the product 3. Provide the reactant and reaction H HO
A: ANSWER 23. Given product is cis-1,2-diol . means it is an example of syn-dihydroxylation , which occ...
Q: Which among these have the highest boiling point? Rank them from A-D. Methanol, water, ethanol, and ...
A: The boiling point of a compound depends upon intermolecular forces and molecular mass. Greater the i...
Q: 1. LIAIH,, (CH,CH,)0, –10C H. 2. H', H0 1 CH3 [1] CH;CH,0 [2] H20 I [1] CH;S- H H. [2] H20
A:
Q: Provide the full CURVED ARROW MECHANISM for the following reaction=
A: Types of intermediates in organic reactions : 1. Carbocation 2. Carbanion 3. Free radical A...
Q: given a stock solution of 2.0%,how would you prepare 10mL of the following solution? find mL stock a...
A:
Q: ion 18 of 20 Consider the Diels-Alder reaction shown. Draw the structures of the diene and dienophil...
A: The correct answer is given below
Q: Which of the following is not a characteristic of a genetic code? a. reading of codons does not inv...
A: a. reading of codons does not involve any overlap This statement is correct.
Q: Which of the following is the most correct name for this compound? a. 2,5-dioxooctanal b. 4,7-dioxoo...
A:
Q: A protein binds a ligand with a KD value of 0.25 M. If you are at a ligand concentration of 0.63 M, ...
A: Fraction of the protein that bound at given ligand concentration is given by: ...
Q: Consider the equilibrium system described by the chemical reaction below. For this reaction, Kc = 2....
A:
Q: The freezing pt. of a solution containing 65g of a compound in 100g benzene is 3.8 degree celcius. w...
A: The depression in freezing point is directly proportional to the molality of the solution. ∆Tf direc...
Q: A solution of tartaric acid (H:C4H.Oe) With a knówn concentration of 0.155 M H:C.H.O. is titrated wi...
A: Answer ) this is acid base titration. In this titration 2 mole of NaoH (base ) required to neutraliz...
Q: If a mixture of solid nickel(II) oxide and 0.13000 M carbon monoxide is allowed to come to equilibri...
A:
Q: Classify K2O strong acid weak acid strong base weak base
A: Acid- The species which is able to donate H+ ion is called as acid. Bade The species which is able ...
Q: For each addition route of the reaction, draw the structure(s) of the major product(s), including st...
A:
Q: Identify the proper form of the equilibrium-constant expression for the equation N2 (g) + O2 (g) = 2...
A:
Q: 11.19 Draw the structures of cis-2-butene and trans-2- butene. Which of the two compounds would give...
A:
Q: Describe (in detail) the preparation of 800 mL of 50 parts per thousand of Cl- from 0.02 M Lead(II) ...
A: Here we have to prepare 800ml of 50 parts per thousand of Cl- from 0.02 M PbCl2 solution.
Q: - of Ke for
A:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- MassPercent1. An oven-cleaning solution is 40.0% NaOH (by mass). If one jar of this product contains 457 g of solution, how many grams of NaOH does it contain? Question options: 274 g 183 g 8.75 g 114 gGodzilla can swim in ocean water because the salt content helps keep him buoyant. The molarity of sodium chloride in sea water is 0.479 M. How many grams of NaCl are there in 2.0 liters of ocean water? Question Blank 6 of 8Answer 3, 4 and 5. Show complete solution with regards to significant figures. The molar mass of the solution is 260.016 g/mol
- Question 9.) What is the molarity when 31.50 grams of potassium chloride is added to water to make a 1.25 L solution? Choose the answer with the appropriate significant figures and choose the correct unit. Choices: 0.338, 25, 338, 25.2, 0.34 Unit: g/L, mol/mL, mM, M, mMolarity Calculations. Report answers with correct units and number of sig figs please. 1. How many grams of NaCl are in 500. mL of a 1.5 M solution? 2. Calculate the molarity of the solution if 72.8g of ethanol, C2H6O, is dissolved in 2.34 L of solution? 3. What volume of 0.200 M ethanol solution contains 1.22 moles of ethanol?A 650.0−g sample of river water taken near an industrial plant contains 27 mg of chromium.What is the mass percent of chromium in the river? How many parts per million of chromium are in the river? How many parts per billion? answer in scientfic notation
- A pharmacist was tasked to prepare 7% glycerin solution by weight.The solution was found to have a density of 1.0149 g/cm3 at 20°C. Glycerin has a molecular weight of 92.0473 g/mol and density of 1.2609g/cm3 at 20°C. Question: 1. What is the weight in g of the solute? 2. What is the moles of solute? 3. What is the weight in kg of the solvent? 4. What is the molality of the solution? 5. What is the molarity of the solution? Show complete solution. Tnx.For the following questions (1-5), consider the compound tartrazine (Yellow 5 food dye) that has a chemical formula of C16H9N4Na3O9S2. Ana weighs out 0.1328 g of tartrazine powder on the balance for her experiment and makes a stock solution by dissolving her powder in 500.0 mL of solution. 1. Determine the number of moles of tartrazine that Ana weighed out. 2. Find the molarity (mol/L) of Ana’s tartrazine solution. 3. Ana makes 5 tartrazine standards by diluting her solution and measures their absorbances using a spectrophotometer: 4. Calculate the molar extinction coefficient (molar absorptivity) of tartrazine in units of 1/(M·cm). Assume a cuvette path length of 1.00 cm. 5. If Ana determines the absorbance of an unknown tartrazine sample to be 0.662, what is the concentration of the sample in µM?0 g of sugar dissolved in 100 g of water. Given: Formula: Solution: Final Answer: 50 mL of water mixed with 20 mL isopropyl alcohol Given: Formula: Solution: Final Answer: How many percent of table salt is present in 662 g salt solution containing 90 g table salt? Given: Formula: Solution: Final Answer: What is the molarity of a solution of NaOH if there are 8.25 grams of NaOH dissolved water to make 3 liters of solution? Given: Formula: Solution: Final Answer: What is the molarity of a solution of NaOH if there are 90 grams of NaOH dissolved water to make 2.5 liters of solution? Given: Formula: Solution: Final Answer:
- QUESTION#1 TOPIC: CALCULATING MOLARITY USING SOLUTE MASS A chemist prepares a solution of potassium iodide (KI) by measuring out 421. g of potassium iodide into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's potassium iodide solution. Round your answer to 3 significant digits. ? mol/LCalculate the molarity of 8.25 mL of 0.0417 M KI diluted to 12.0 mL with water. Answer in M, not in scientific notationQUESTION 42 What is the molarity of MgCl 2 in 325 mL of solution that contains 15.6g MgCl 2? 0.504 M 48 M 5.04 x 10-3 M 53.3 M

