Determine the intermolecular forces for each of the following molecules: Compound N₂ CH4 CH3CI H₂O HF CCl4 NH3 NF3 total valance electrons Lewis Structure Molecular Geometry For a molecule to be polar, it must satisfy the following two requirements: 1. The molecule must have polar bonds 2. The molecule must have a lack of symmetry, so the bond dipoles do not cancel each other. The lack of symmetry will exist if the central atom is bonded to different elements, and/or contains at least one lone pair. 1. Does the molecule contain polar bonds? yes or no? 2. Does the molecule have a lack of symmetry? yes or no? Is the molecule polar? yes or no? Dominant Intermolecular Force for Molecul
Determine the intermolecular forces for each of the following molecules: Compound N₂ CH4 CH3CI H₂O HF CCl4 NH3 NF3 total valance electrons Lewis Structure Molecular Geometry For a molecule to be polar, it must satisfy the following two requirements: 1. The molecule must have polar bonds 2. The molecule must have a lack of symmetry, so the bond dipoles do not cancel each other. The lack of symmetry will exist if the central atom is bonded to different elements, and/or contains at least one lone pair. 1. Does the molecule contain polar bonds? yes or no? 2. Does the molecule have a lack of symmetry? yes or no? Is the molecule polar? yes or no? Dominant Intermolecular Force for Molecul
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.43PAE: 8.43 Identify the kinds of intermolecular forces (London dispersion, dipoledipole, or hydrogen...
Related questions
Question
Need help
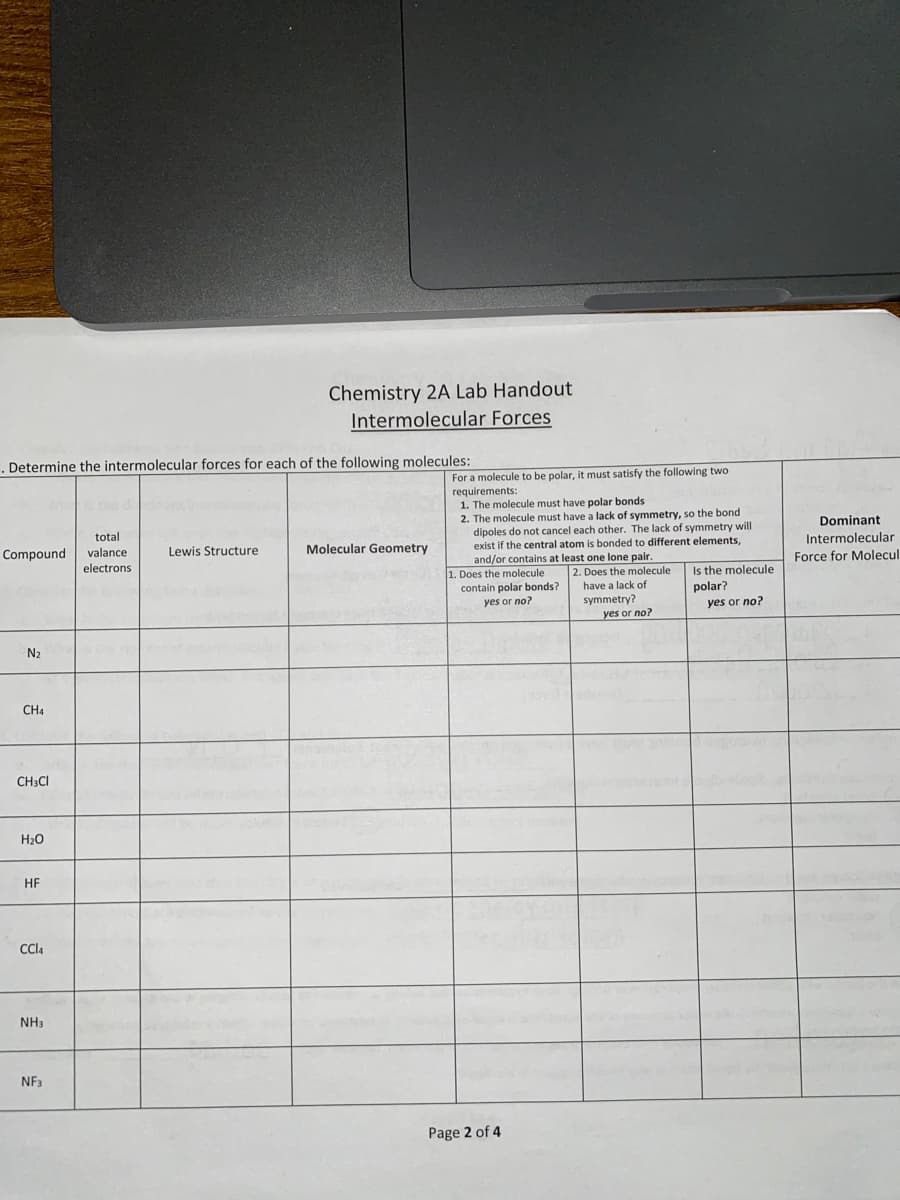
Transcribed Image Text:. Determine the intermolecular forces for each of the following molecules:
Compound
2
N₂
CH4
CH3CI
H₂O
HF
CCl4
NH3
NF3
total
valance.
electrons
Chemistry 2A Lab Handout
Intermolecular Forces
Lewis Structure
Molecular Geometry
For a molecule to be polar, it must satisfy the following two
requirements:
1. The molecule must have polar bonds
2. The molecule must have a lack of symmetry, so the bond
dipoles do not cancel each other. The lack of symmetry will
exist if the central atom is bonded to different elements,
and/or contains at least one lone pair.
1. Does the molecule
contain polar bonds?
yes or no?
2. Does the molecule
have a lack of
symmetry?
yes or no?
Page 2 of 4
Is the molecule
polar?
yes or no?
Dominant
Intermolecular
Force for Molecul
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax