em 105-Online TA TABLE Mass of test tube Mass of test tube & metal Mass of metal Mass of calorimeter Mass of calorimeter & water Mass of water Temp of boiling water (metal) Temperature of water in foam cup Highest temp (after metal added) Change in water temperature (ATW) Change in metal temperature (ATM) Trial 1 19.34g 38.14g 18.8g 3.819 51.709 47.99 206°F 98°C 72.5°f 23°C 80 of 26°C 1 Post-lab Calculations: 1. Calculate the specific heat, for both trials, by setting heat gained by (qw) to heat lost by the metal (qm). Trial 1 : Trial 2:
em 105-Online TA TABLE Mass of test tube Mass of test tube & metal Mass of metal Mass of calorimeter Mass of calorimeter & water Mass of water Temp of boiling water (metal) Temperature of water in foam cup Highest temp (after metal added) Change in water temperature (ATW) Change in metal temperature (ATM) Trial 1 19.34g 38.14g 18.8g 3.819 51.709 47.99 206°F 98°C 72.5°f 23°C 80 of 26°C 1 Post-lab Calculations: 1. Calculate the specific heat, for both trials, by setting heat gained by (qw) to heat lost by the metal (qm). Trial 1 : Trial 2:
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.32E: Many compressed gases come in large,heavy metal cylindersthat are so heavy that they need a special...
Related questions
Question
100%
Please help with the last 2 boxes in trial 1
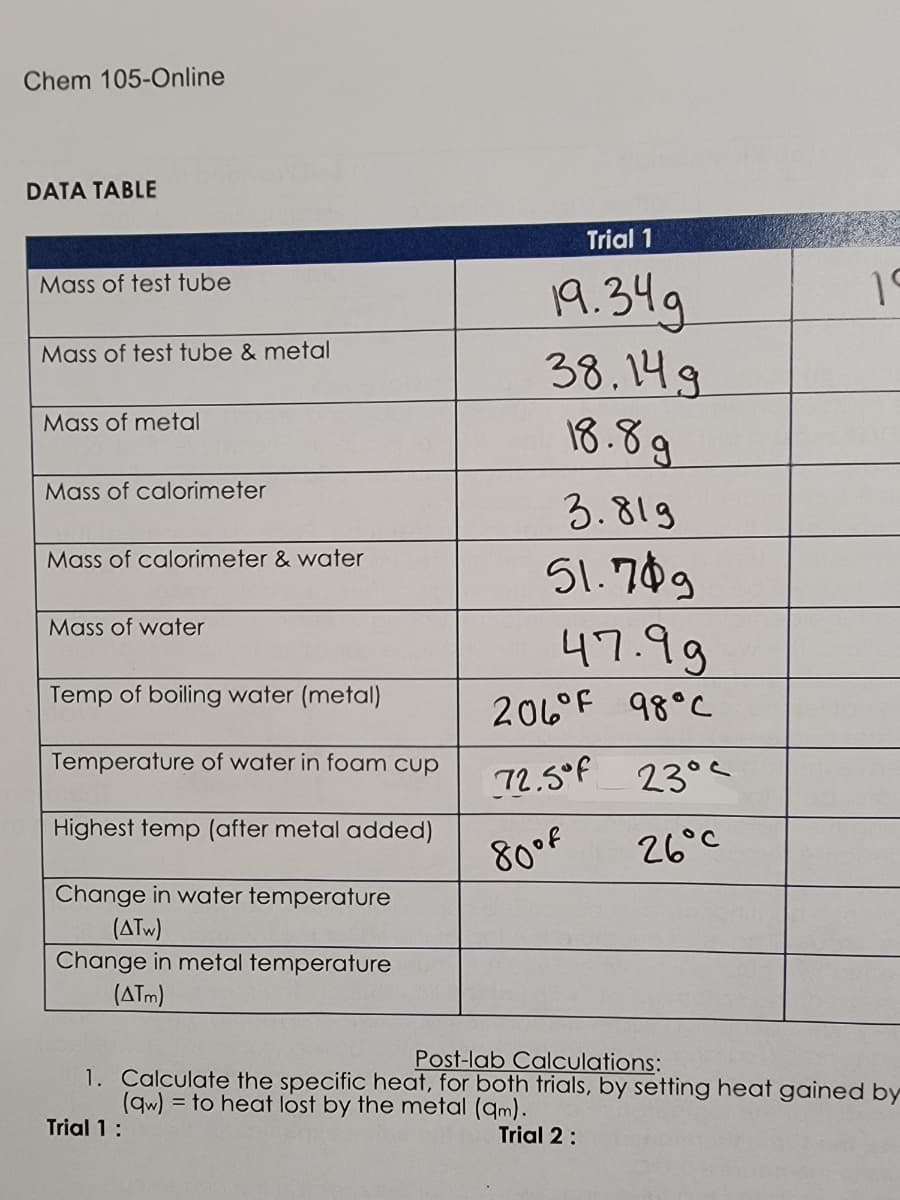
Transcribed Image Text:Chem 105-Online
DATA TABLE
Mass of test tube
Mass of test tube & metal
Mass of metal
Mass of calorimeter
Mass of calorimeter & water
Mass of water
Temp of boiling water (metal)
Temperature of water in foam cup
Highest temp (after metal added)
Change in water temperature
(ATW)
Change in metal temperature
(ATM)
Trial 1
19.349
38.14.g
18.8g
3.819
51.789
47.99
206°F 98°C
72.5°F 23°C
80°f
26°C
19
Post-lab Calculations:
1. Calculate the specific heat, for both trials, by setting heat gained by
(qw) to heat lost by the metal (qm).
Trial 1:
Trial 2:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,