Determine the Kb for an unknown base by constructing an ICE table and using this information to construct and solve the equilibrium constant expression. Complete Parts 1-2 before submitting your answer. 1 2 NEXT > The pH for a 0.185 M solution of an unknown weak base, B, is 12.95. Fill in the ICE table with the appropriate value for each involved species to determine the unknown concentrations of all reactants and products. Initial (M) Change (M) Equilibrium (M) B(aq) H₂O(1) = OH-(aq) + BH-(aq) RESET 0 0.185 12.95 -12.95 1.11 -1.11 1.12 x 10-13
Determine the Kb for an unknown base by constructing an ICE table and using this information to construct and solve the equilibrium constant expression. Complete Parts 1-2 before submitting your answer. 1 2 NEXT > The pH for a 0.185 M solution of an unknown weak base, B, is 12.95. Fill in the ICE table with the appropriate value for each involved species to determine the unknown concentrations of all reactants and products. Initial (M) Change (M) Equilibrium (M) B(aq) H₂O(1) = OH-(aq) + BH-(aq) RESET 0 0.185 12.95 -12.95 1.11 -1.11 1.12 x 10-13
Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.22QAP
Related questions
Question
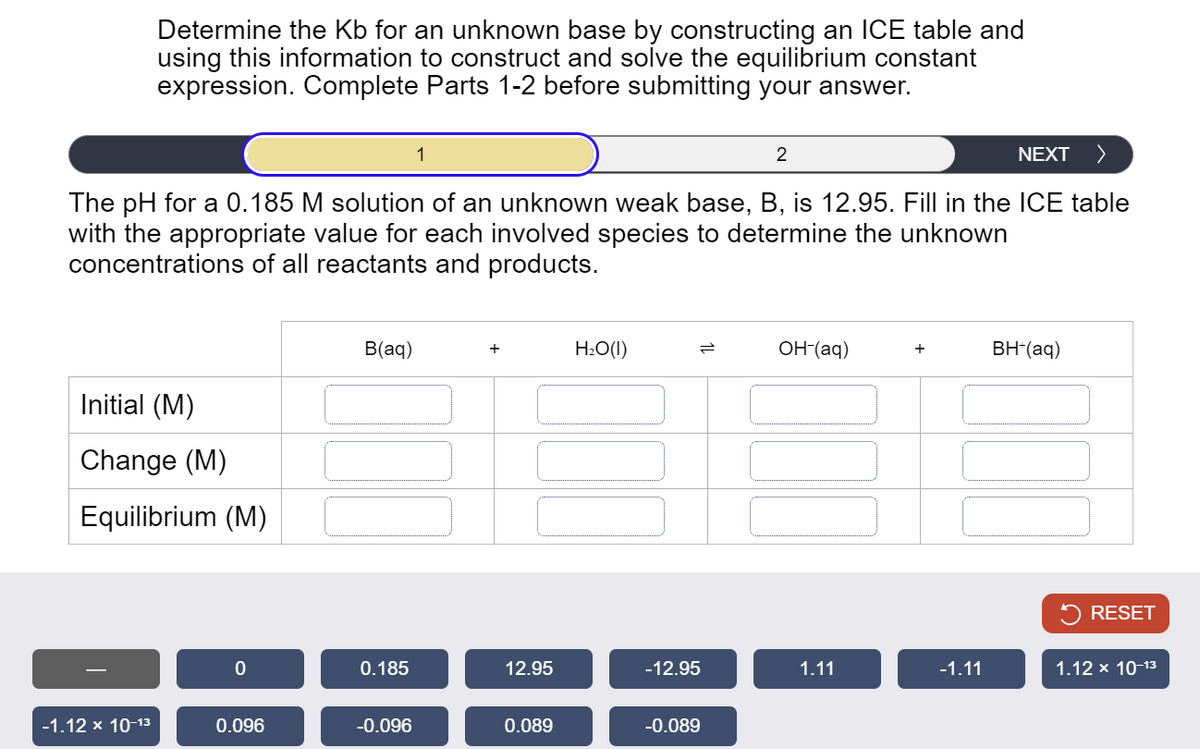
Transcribed Image Text:Determine the Kb for an unknown base by constructing an ICE table and
using this information to construct and solve the equilibrium constant
expression. Complete Parts 1-2 before submitting your answer.
1
2
NEXT >
The pH for a 0.185 M solution of an unknown weak base, B, is 12.95. Fill in the ICE table
with the appropriate value for each involved species to determine the unknown
concentrations of all reactants and products.
Initial (M)
Change (M)
Equilibrium (M)
B(aq)
H₂O(1)
=
OH-(aq)
+
BH+(aq)
RESET
0
0.185
12.95
-12.95
1.11
-1.11
1.12 × 10-13
-1.12 × 10-13
0.096
-0.096
0.089
-0.089
![Determine the Kb for an unknown base by constructing an ICE table and
using this information to construct and solve the equilibrium constant
expression. Complete Parts 1-2 before submitting your answer.
PREV
1
2
Based on your ICE table (Part 1) and the definition of Kb, set up the expression for Kb.
Each reaction participant must be represented by one tile. Do not combine terms.
Once the expression is constructed, solve for Kb.
Kb
=
=
RESET
[0]
[0.185]
[12.95]
[1.11]
[1.12 × 10-13]
[0.096]
[0.089]
6.8 × 10-26
1 × 10-14
0.083
6.1 x 10-13
1.3 x 10-25
12](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff922ab2d-c754-4c9c-9d3b-bd784fecfd89%2Fb6b0c3e7-00cf-4e53-8d45-bb29324a40aa%2Fjzpycaq_processed.png&w=3840&q=75)
Transcribed Image Text:Determine the Kb for an unknown base by constructing an ICE table and
using this information to construct and solve the equilibrium constant
expression. Complete Parts 1-2 before submitting your answer.
PREV
1
2
Based on your ICE table (Part 1) and the definition of Kb, set up the expression for Kb.
Each reaction participant must be represented by one tile. Do not combine terms.
Once the expression is constructed, solve for Kb.
Kb
=
=
RESET
[0]
[0.185]
[12.95]
[1.11]
[1.12 × 10-13]
[0.096]
[0.089]
6.8 × 10-26
1 × 10-14
0.083
6.1 x 10-13
1.3 x 10-25
12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning