Determine the mass (in grams) of magnesium metal required to produce 40.0 mL of H2with a temperature of 26.9 oC and an atmospheric pressure of 745 mmHg. Be sure to take into account water vapour using equation (3). Equation (3): See Image
Determine the mass (in grams) of magnesium metal required to produce 40.0 mL of H2with a temperature of 26.9 oC and an atmospheric pressure of 745 mmHg. Be sure to take into account water vapour using equation (3). Equation (3): See Image
Chapter9: Aqueous Solutions And Chemical Equilibria
Section: Chapter Questions
Problem 9.20QAP
Related questions
Question
100%
Determine the mass (in grams) of magnesium metal required to produce 40.0 mL of H2with a temperature of 26.9 oC and an atmospheric pressure of 745 mmHg. Be sure to take into account water vapour using equation (3).
Equation (3): See Image
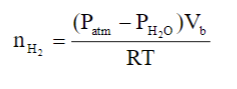
Transcribed Image Text:(Pam – PH,0)V,
RT
||
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

