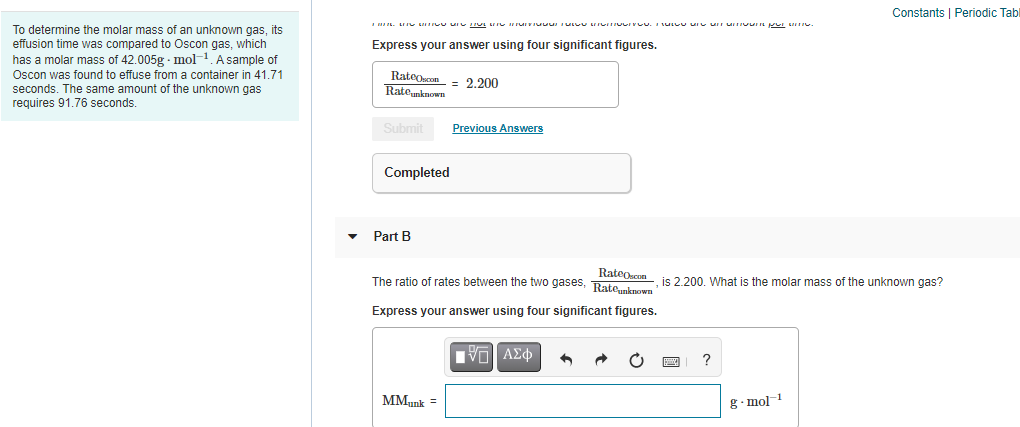
determine the molar mass of an unknown gas, its usion time was compared to Oscon gas, which sa molar mass of 42.005g - mol-1. A sample of scon was found to effuse from a container in 41.71 Express your answer using four significant figures. RateOscon Ratemknown 2.200 conds. The same amount of the unknown gas quires 91.76 seconds. Submit Previous Answers Completed Part B RateOscon Rateunknown The ratio of rates between the two gases, ,is 2.200. What is the molar mass of the unknown gas? Express your answer using four significant figures. ?
determine the molar mass of an unknown gas, its usion time was compared to Oscon gas, which sa molar mass of 42.005g - mol-1. A sample of scon was found to effuse from a container in 41.71 Express your answer using four significant figures. RateOscon Ratemknown 2.200 conds. The same amount of the unknown gas quires 91.76 seconds. Submit Previous Answers Completed Part B RateOscon Rateunknown The ratio of rates between the two gases, ,is 2.200. What is the molar mass of the unknown gas? Express your answer using four significant figures. ?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter10: Gases And Their Properties
Section: Chapter Questions
Problem 83GQ: Carbon dioxide, CO2, was shown lo effuse through a porous plate at the rate of 0.033 mol/min. The...
Related questions
Question
Transcribed Image Text:Constants | Periodic Tabl
To determine the molar mass of an unknown gas, its
effusion time was compared to Oscon gas, which
has a molar mass of 42.005g - mol-1. A sample of
Express your answer using four significant figures.
Oscon was found to effuse from a container in 41.71
RateOscon
Rateunknown
= 2.200
seconds. The same amount of the unknown gas
requires 91,76 seconds.
Submit
Previous Answers
Completed
Part B
RateOscon
The ratio of rates between the two gases, Rate.known
is 2.200. What is the molar mass of the unknown gas?
Express your answer using four significant figures.
?
MMunk
g. mol 1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning