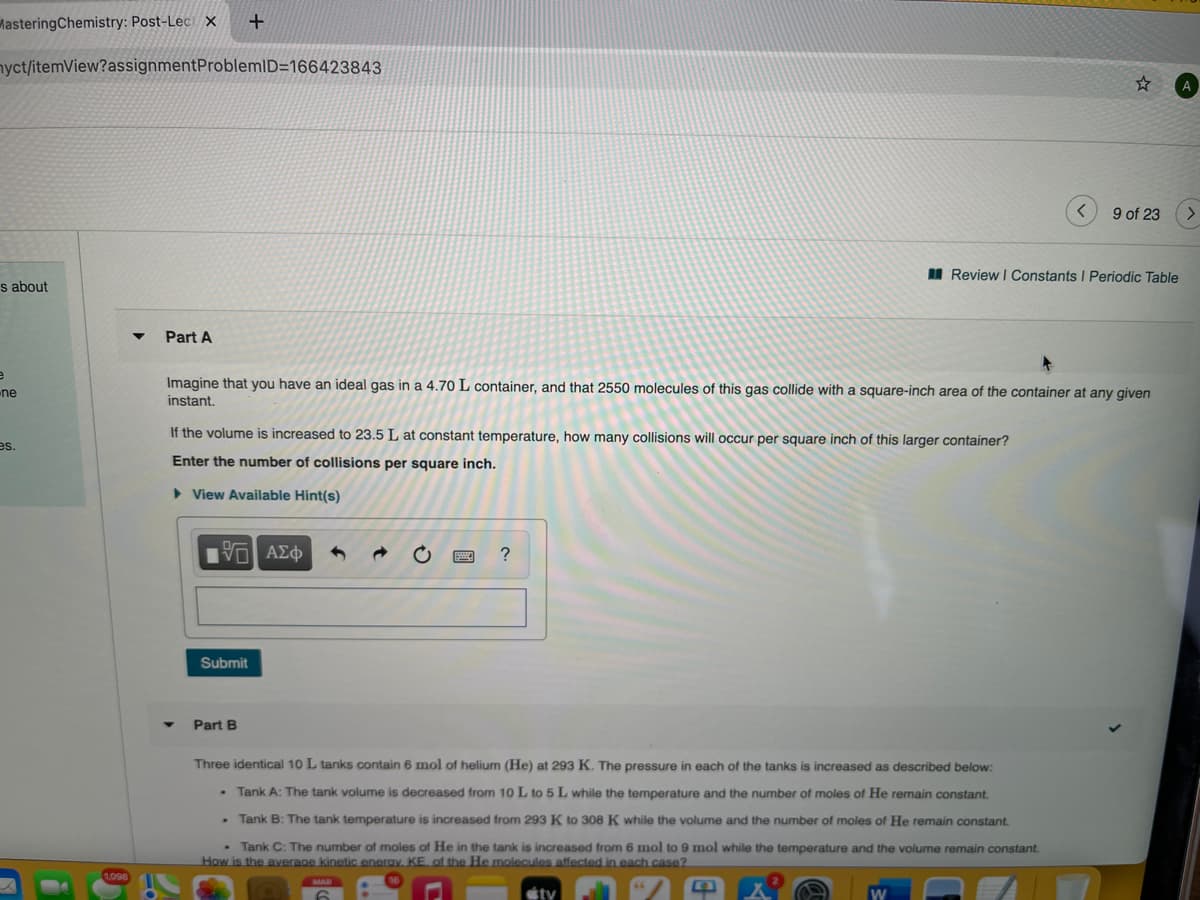
Imagine that you have an ideal gas in a 4.70 L container, and that 2550 molecules of this gas collide with a square-inch area of the container at any given instant. If the volume is increased to 23.5 L at constant temperature, how many collisions will occur per square inch of this larger container? Enter the number of collisions per square inch. > View Available Hint(s) Submit
Imagine that you have an ideal gas in a 4.70 L container, and that 2550 molecules of this gas collide with a square-inch area of the container at any given instant. If the volume is increased to 23.5 L at constant temperature, how many collisions will occur per square inch of this larger container? Enter the number of collisions per square inch. > View Available Hint(s) Submit
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter5: Gases
Section: Chapter Questions
Problem 130E
Related questions
Question
100%
Transcribed Image Text:MasteringChemistry: Post-Lec ×
hyct/itemView?assignmentProblemlD=166423843
A
9 of 23
I Review I Constants I Periodic Table
s about
Part A
Imagine that you have an ideal gas in a 4.70 L container, and that 2550 molecules of this gas collide with a square-inch area of the container at any given
ne
instant.
If the volume is increased to 23.5 L at constant temperature, how many collisions will occur per square inch of this larger container?
es.
Enter the number of collisions per square inch.
> View Available Hint(s)
ΑΣφ
Submit
Part B
Three identical 10 L tanks contain 6 mol of helium (He) at 293 K. The pressure in each of the tanks is increased as described below:
• Tank A: The tank volume is decreased from 10 L to 5 L while the temperature and the number of moles of He remain constant.
• Tank B: The tank temperature is increased from 293 K to 308 K while the volume and the number of moles of He remain constant.
• Tank C: The number of moles of He
How is the average kinetic enerav. KE of the He molecules affected in each case?
the tank is increased from 6 mol to 9 mol while the temperature and the volume remain constant.
MAR
stv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning