Determine the molecular and empirical formulas for the substance shown in the ball-and-stick model below. S= yellow Cl = green Molecular Formula Empirical Formula Submit Answer Retry Entire Group 3 more group attempts remaining
Determine the molecular and empirical formulas for the substance shown in the ball-and-stick model below. S= yellow Cl = green Molecular Formula Empirical Formula Submit Answer Retry Entire Group 3 more group attempts remaining
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 21ALQ: True or false? The atom with the largest subscript in a formula is the atom with the largest percent...
Related questions
Question
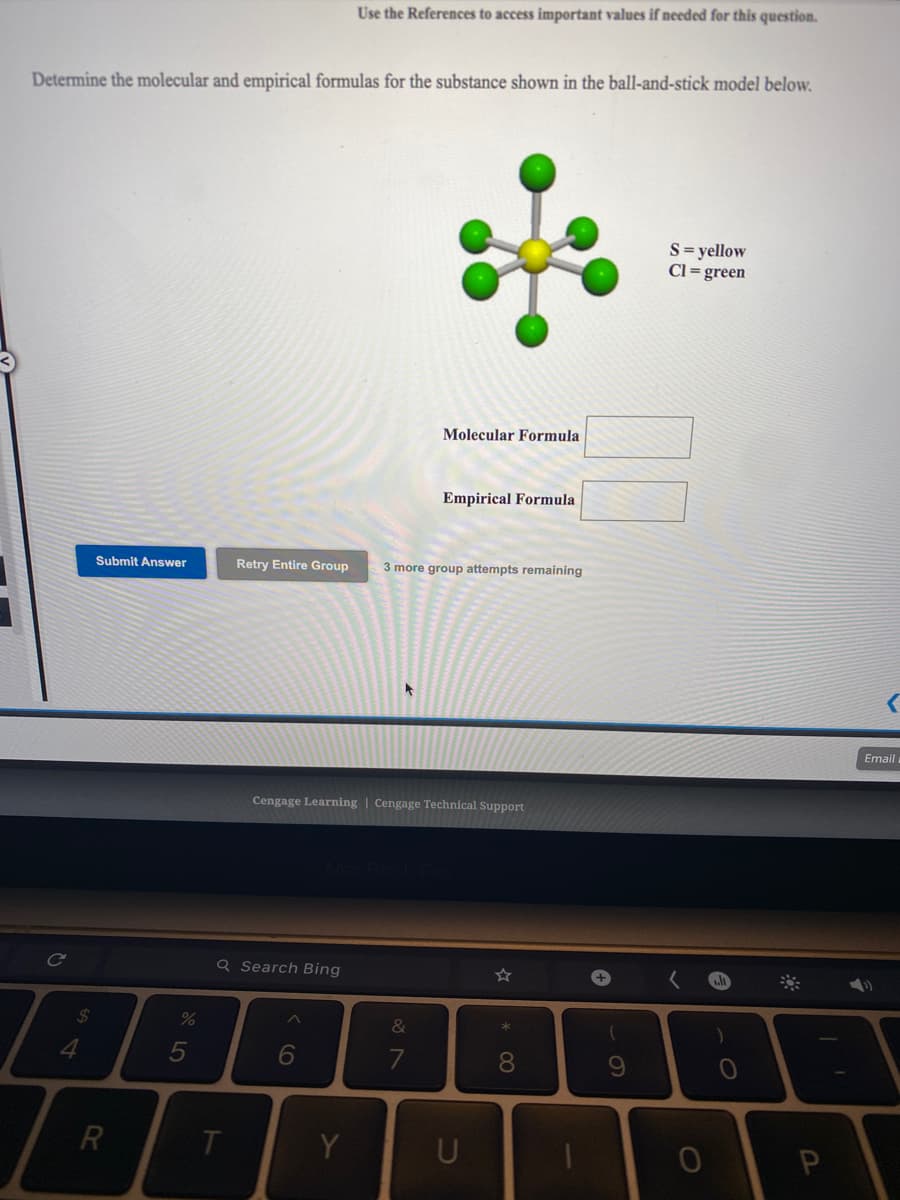
Transcribed Image Text:Use the References to access important values if needed for this question.
Determine the molecular and empirical formulas for the substance shown in the ball-and-stick model below.
S= yellow
Cl= green
Molecular Formula
Empirical Formula
Submit Answer
Retry Entire Group
3 more group attempts remaining
Email
Cengage Learning | Cengage Technical Support
Ce
Q Search Bing
24
%
&
4.
5
6
7
T.
Y
U
* CO
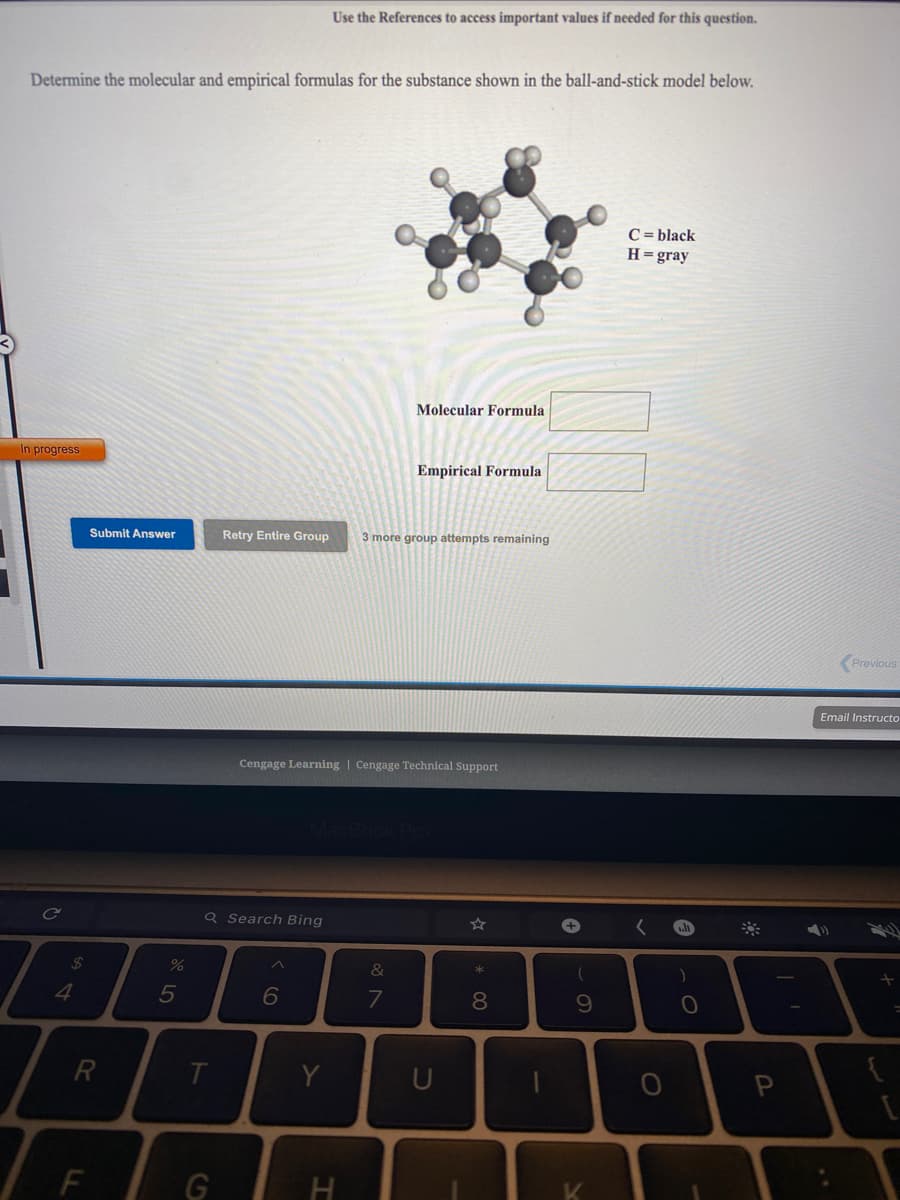
Transcribed Image Text:Use the References to access important values if needed for this question.
Determine the molecular and empirical formulas for the substance shown in the ball-and-stick model below.
C = black
H=gray
Molecular Formula
In progress
Empirical Formula
Submit Answer
Retry Entire Group
3 more group attempts remaining
Previous
Email Instructo
Cengage Learning | Cengage Technical Support
Q Search Bing
%24
%
&
5
7
8.
R
Y
U
F.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning