Table 1. Reaction of steel wool in presence of oxygen. Complete the following table. Remember sigfigs! Sample 1 Sample 2 Sample 3 Mass steel wool (g) 2.68 2.20 2.89 Mass compound (g) 2.98 2.50 3.14 Mass oxygen added (g) 0.30 0.30 0.25 Moles Fe 0.048 0.039 0.052 Moles O 0.02 0.02 0.016 Molecular Formula "ost-lab Questions 1. What is the empirical formula for the iron oxide in this experiment? Show all work at the end of the worksheet. Click or tap here to enter text. 2. Does your empirical formula agree with the expected empirical formula? The expected form of iron oxide in rust is iron(III) oxide.
Table 1. Reaction of steel wool in presence of oxygen. Complete the following table. Remember sigfigs! Sample 1 Sample 2 Sample 3 Mass steel wool (g) 2.68 2.20 2.89 Mass compound (g) 2.98 2.50 3.14 Mass oxygen added (g) 0.30 0.30 0.25 Moles Fe 0.048 0.039 0.052 Moles O 0.02 0.02 0.016 Molecular Formula "ost-lab Questions 1. What is the empirical formula for the iron oxide in this experiment? Show all work at the end of the worksheet. Click or tap here to enter text. 2. Does your empirical formula agree with the expected empirical formula? The expected form of iron oxide in rust is iron(III) oxide.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.99PAE: Substances that poison a catalyst pose a major concern for many engineering designs, including those...
Related questions
Question
Molecular formula for the 3 samples and question 1,2. Thanks
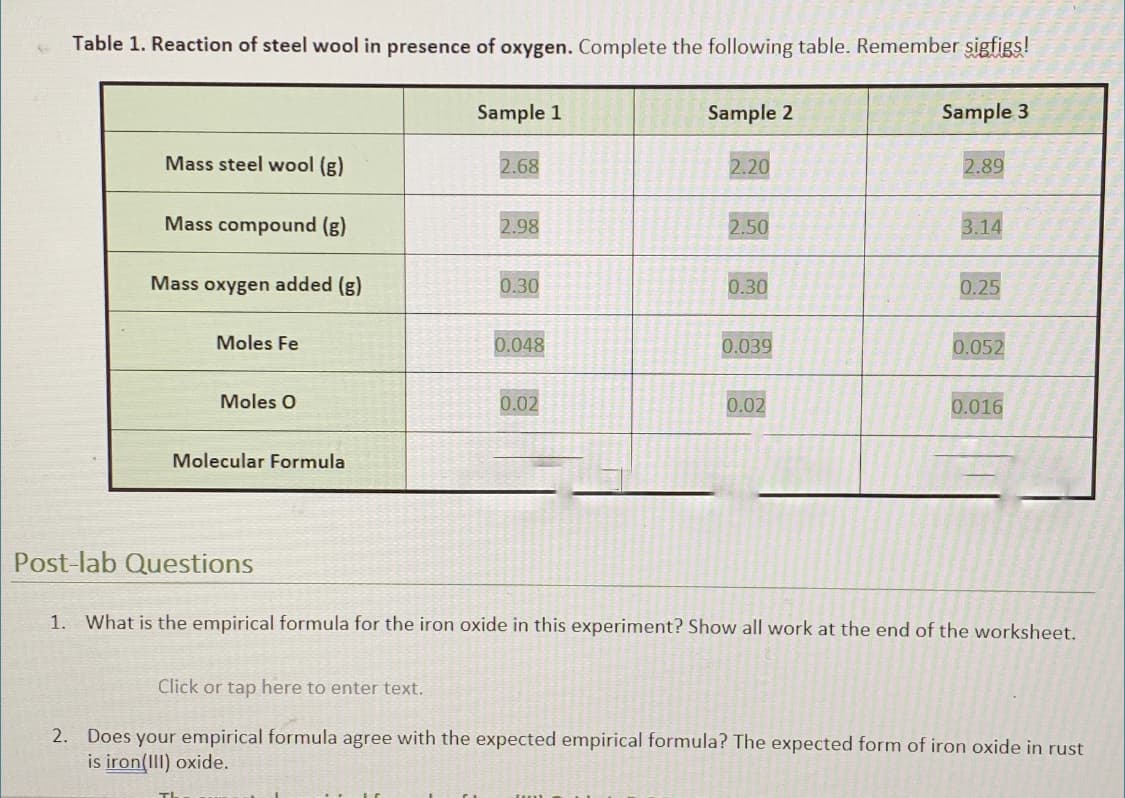
Transcribed Image Text:Table 1. Reaction of steel wool in presence of oxygen. Complete the following table. Remember sigfigs!
Sample 1
Sample 2
Sample 3
Mass steel wool (g)
2.68
2.20
2.89
Mass compound (g)
2.98
2.50
3.14
Mass oxygen added (g)
0.30
0.30
0.25
Moles Fe
0.048
0.039
0.052
Moles O
0.02
0.02
0.016
Molecular Formula
Post-lab Questions
1. What is the empirical formula for the iron oxide in this experiment? Show all work at the end of the worksheet.
Click or tap here to enter text.
2. Does your empirical formula agree with the expected empirical formula? The expected form of iron oxide in rust
is iron(III) oxide.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
