Determine the number of valence electrons in SiF, and then draw the corresponding Lewis structure. Incorrect, 2 attempts remaining Your submission: Feedback: C) 32 Correct number of valence electrons You have submitted an incorrect structure due to a violation of the octet rule. Fluorine cannot have ドー -F Si. less than 8 valence electrons.
Determine the number of valence electrons in SiF, and then draw the corresponding Lewis structure. Incorrect, 2 attempts remaining Your submission: Feedback: C) 32 Correct number of valence electrons You have submitted an incorrect structure due to a violation of the octet rule. Fluorine cannot have ドー -F Si. less than 8 valence electrons.
Chapter2: Resonance Structures
Section: Chapter Questions
Problem 27EQ
Related questions
Question
100%
I do not understand this question
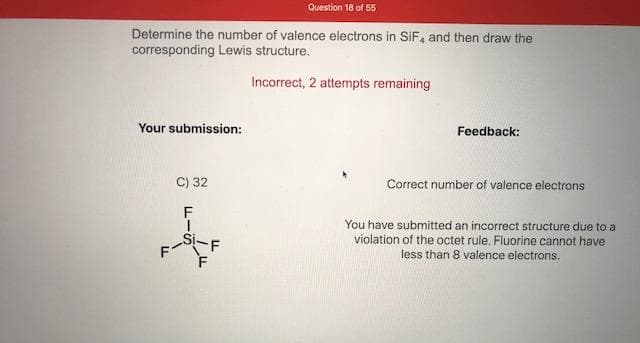
Transcribed Image Text:Determine the number of valence electrons in SiF, and then draw the
corresponding Lewis structure.
Incorrect, 2 attempts remaining
Your submission:
Feedback:
C) 32
Correct number of valence electrons
You have submitted an incorrect structure due to a
violation of the octet rule. Fluorine cannot have
ドー
-F
Si.
less than 8 valence electrons.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
