Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 49RGQ: Fluoridation of city water supplies has been practiced in the United States for several decades. It...
Related questions
Question

Transcribed Image Text:of a 0.9% solution of sodium chloride
(NaCI), we would weigh 0.9 g of NaCl (solute) and dissolve
it in some distilled wate (solvent). When the salt is totally
dissolved, enough distilled water is added to make a final volume
of 100 mL. When measuring solutions in a cylinder, pipette,
or volumetric flask, be sure to hold the calibrated glassware at
eye level to read the bottom line of the curved surface of the
liquid (the meniscus). Volumetric glassware should be used fo
solution preparation; calibrated Erlenmeyer flasks or beakers
too inaccurate. The size of calibrated glassware chosen shoul
equal to or just slightly larger than the volume desired so tha
error of measurement will be minimal.
Problems
Determine the percentage solution
1. 0.25 g sodium chloride diluted to 100 mL =
2. 6.4 g sodium chloride diluted to 100 mL =
4.0 mL hydrochloric acid diluted to 100 mL = L
4. 0.6 mL nitric acid diluted to 100 mL =
%3D
3.
%3D
5. 20 g of silver nitrate diluted to 100 mL =
6. 2.25 g of Alcian blue diluted to 200 mL =
%3D
To prepare a definite amount of a percentage solutic
following ratio:
multiply
% desired:100 = g or mL needed:volume desire
L multiply -
Expert Solution
Step 1
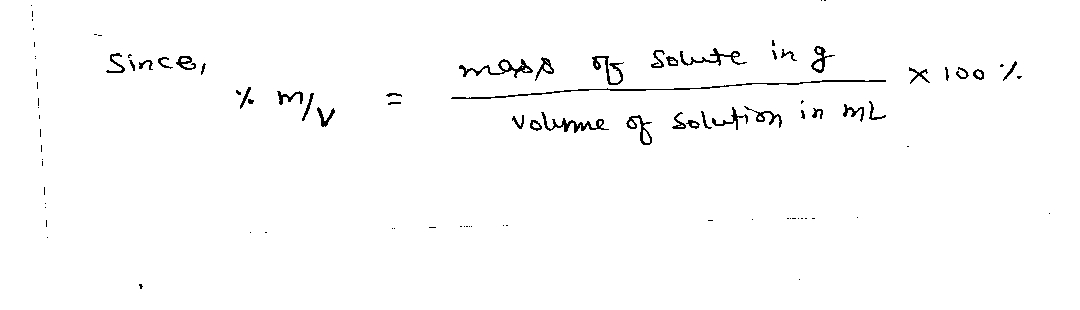
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning