Determine the pH of a solution by constructing a BCA table, constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the pH. The value of Ka for HCIO is 2.9 × 108. Complete Parts 1-4 before submitting your answer. 1 2 4 NEXT > 0.003 mol of solid NaOH is added to a 100.0 mL buffer containing 0.13 M HCIO and 0.37 M NaCIO. Fill in the table with the appropriate value for each involved species to determine the moles of reactant and product after the reaction of the acid and base. You can ignore the amount of water in the reaction. HCIO(aq) + OH(aq) 0.013 0.003 Before (mol) Change (mol) -0.013 0.013 After (mol) 0.010 0.003 H₂O(I) + CIO (aq) 0.037 0.003 0.040 RESET 0 0.13 0.37 0.003 -0.003 0.013 -0.013 0.037 -0.037 0.010 -0.010 0.040 -0.040
Determine the pH of a solution by constructing a BCA table, constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the pH. The value of Ka for HCIO is 2.9 × 108. Complete Parts 1-4 before submitting your answer. 1 2 4 NEXT > 0.003 mol of solid NaOH is added to a 100.0 mL buffer containing 0.13 M HCIO and 0.37 M NaCIO. Fill in the table with the appropriate value for each involved species to determine the moles of reactant and product after the reaction of the acid and base. You can ignore the amount of water in the reaction. HCIO(aq) + OH(aq) 0.013 0.003 Before (mol) Change (mol) -0.013 0.013 After (mol) 0.010 0.003 H₂O(I) + CIO (aq) 0.037 0.003 0.040 RESET 0 0.13 0.37 0.003 -0.003 0.013 -0.013 0.037 -0.037 0.010 -0.010 0.040 -0.040
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 74QAP: Fifty cm3 of 1.000 M nitrous acid is titrated with 0.850 M NaOH. What is the pH of the solution (a)...
Related questions
Question
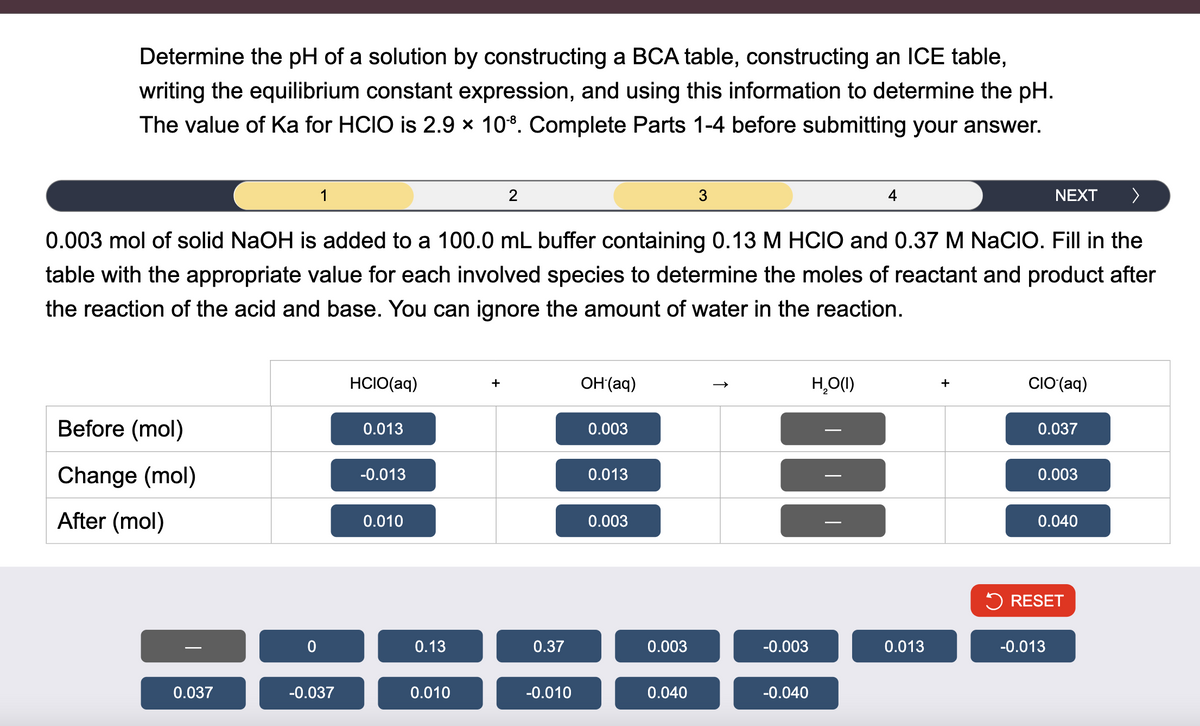
Transcribed Image Text:Determine the pH of a solution by constructing a BCA table, constructing an ICE table,
writing the equilibrium constant expression, and using this information to determine the pH.
The value of Ka for HCIO is 2.9 × 108. Complete Parts 1-4 before submitting your answer.
1
2
4
NEXT >
0.003 mol of solid NaOH is added to a 100.0 mL buffer containing 0.13 M HCIO and 0.37 M NaCIO. Fill in the
table with the appropriate value for each involved species to determine the moles of reactant and product after
the reaction of the acid and base. You can ignore the amount of water in the reaction.
HCIO(aq)
+
OH(aq)
0.013
0.003
Before (mol)
Change (mol)
-0.013
0.013
After (mol)
0.010
0.003
H₂O(I)
+
CIO (aq)
0.037
0.003
0.040
RESET
0
0.13
0.37
0.003
-0.003
0.013
-0.013
0.037
-0.037
0.010
-0.010
0.040
-0.040
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
