Chapter5: Chemical Reactions
Section: Chapter Questions
Problem 5.4E
Related questions
Question
100%
I would like help solving this problem in unit conversion fashion.
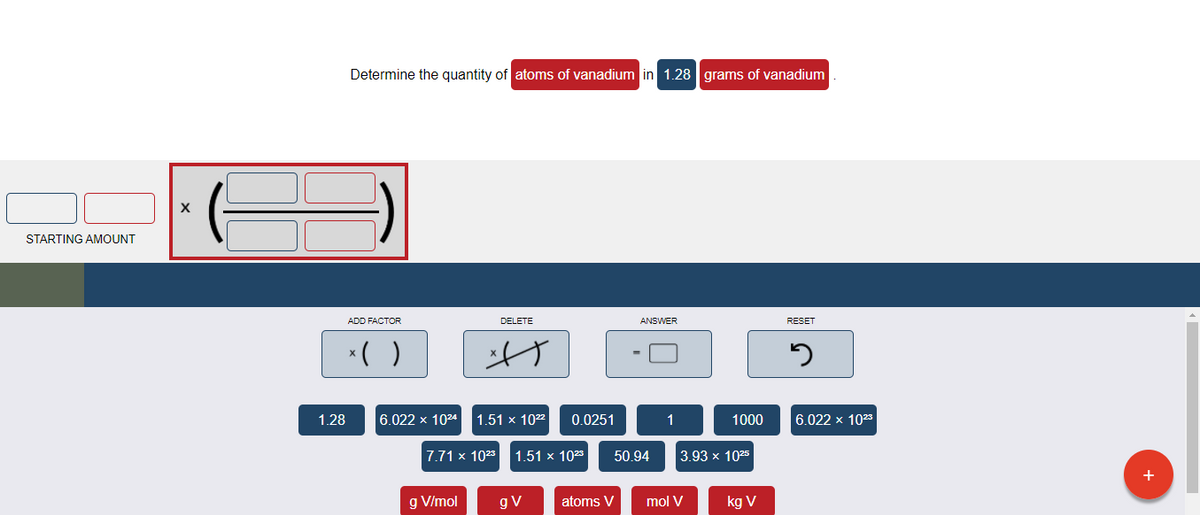
Transcribed Image Text:Determine the quantity of atoms of vanadium in 1.28 grams of vanadium
STARTING AMOUNT
ADD FACTOR
DELETE
ANSWER
RESET
*( )
1.28
6.022 x 1024
1.51 x 1022
0.0251
1000
6.022 x 1023
7.71 × 1023
1.51 x 1023
50.94
3.93 x 1025
+
g V/mol
gV
atoms V
mol V
kg V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning