Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter18: Representative Metals, Metalloids, And Nonmetals
Section: Chapter Questions
Problem 58E: Heating a sample of Na2CO3xH2O weighing 4.640 g until the removal of the water of hydration leaves...
Related questions
Question
Transcribed Image Text:OWLV2 | Online
Students
A 21/FA-CHEM-1110L-03-CHEMIC X
east.cengagenow.com/ilrn/takeAssignment/takeCXPCompliantActivity.do?lo-
CHAPTER 2 - ATOMS, MOLECULES, AND IONS
O Previous
Page 2 of 5
Next O
R
Use the References to access imp
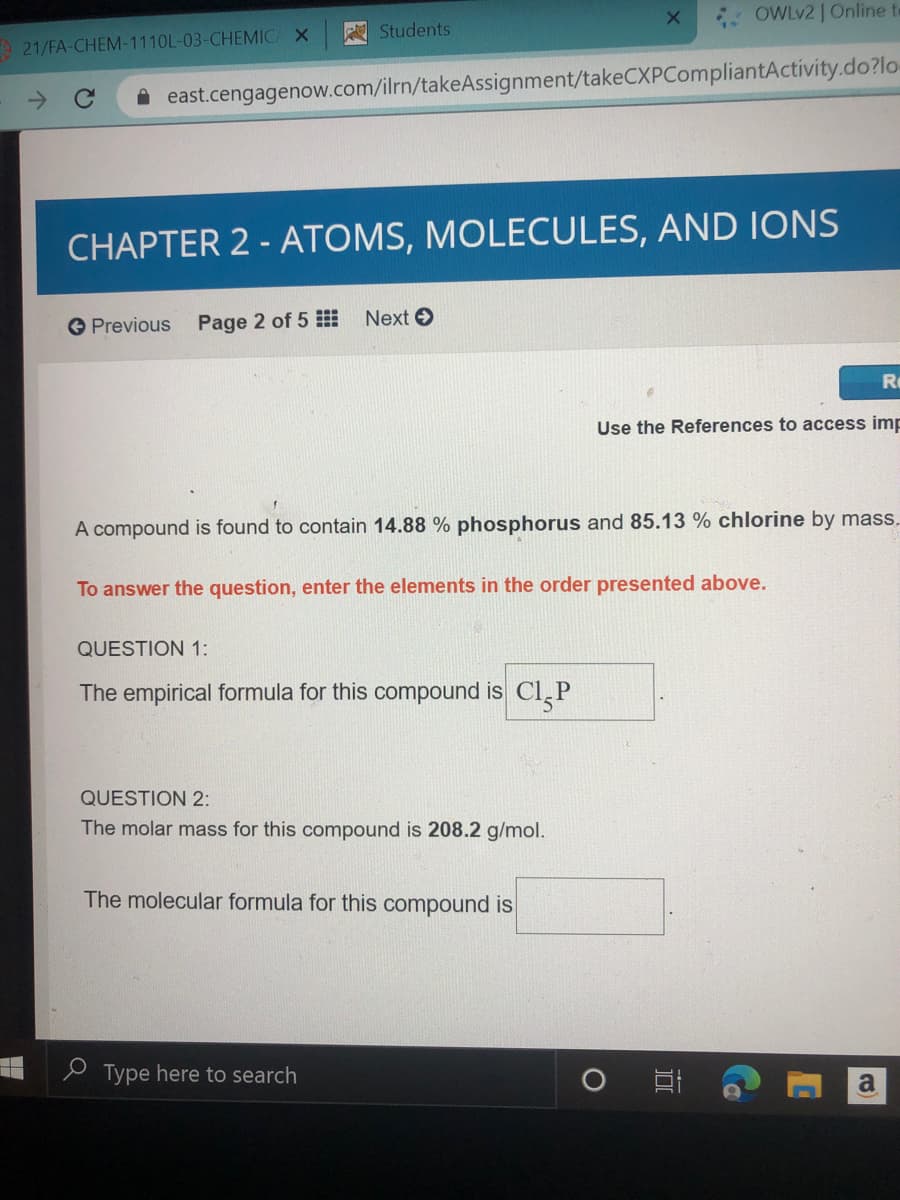
A compound is found to contain 14.88 % phosphorus and 85.13 % chlorine by mass.
To answer the question, enter the elements in the order presented above.
QUESTION 1:
The empirical formula for this compound is Cl,P
QUESTION 2:
The molar mass for this compound is 208.2 g/mol.
The molecular formula for this compound is
Type here to search
a
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning