Determine the total molecular partition function for gaseous H2O at 1000. K confined to a volume of 2.20 cm³. The rotational constants for water are BA = 27.8 cm=', BB = 14.5 cm¬', and Bc = 9.95 cm=. The vibrational frequencies are 1615, 3694, and 3802 cm. The ground electronic state is nondegenerate. (Note: the Avogadro's constant NA = 6.022 × 1023 mol¬1). %3D Express your answer to three significant figures. ΑΣφ ?
Determine the total molecular partition function for gaseous H2O at 1000. K confined to a volume of 2.20 cm³. The rotational constants for water are BA = 27.8 cm=', BB = 14.5 cm¬', and Bc = 9.95 cm=. The vibrational frequencies are 1615, 3694, and 3802 cm. The ground electronic state is nondegenerate. (Note: the Avogadro's constant NA = 6.022 × 1023 mol¬1). %3D Express your answer to three significant figures. ΑΣφ ?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
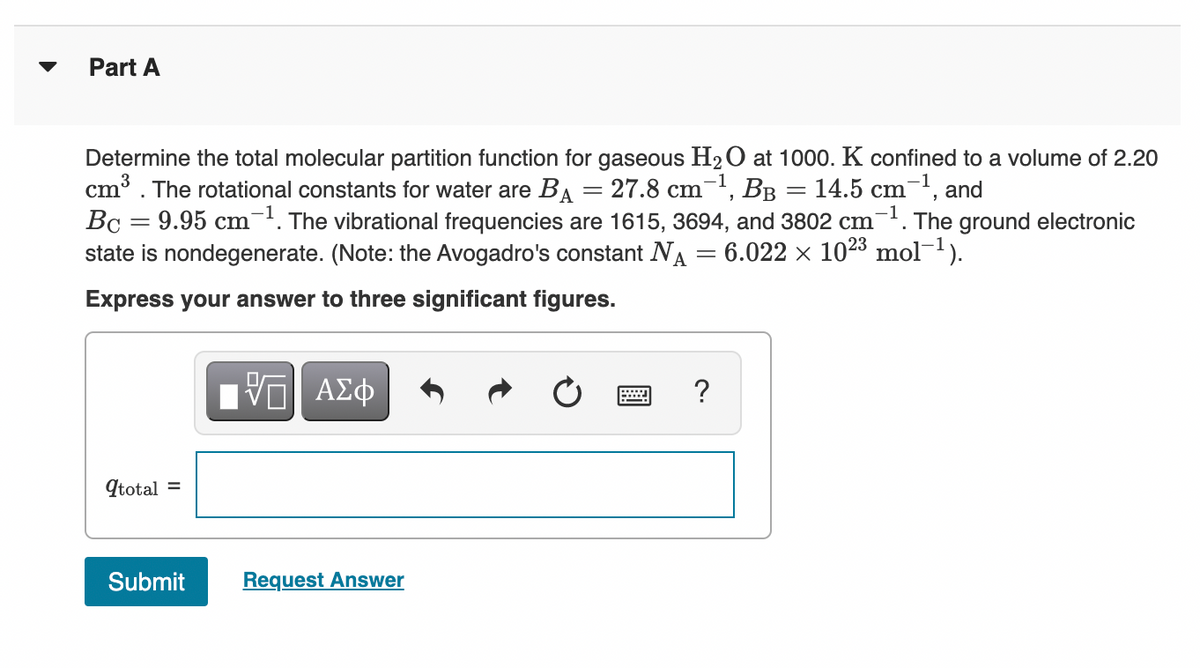
Transcribed Image Text:Part A
Determine the total molecular partition function for gaseous H2O at 1000. K confined to a volume of 2.20
cm³. The rotational constants for water are BA = 27.8 cm, BB = 14.5 cm¯', and
Bc = 9.95 cm. The vibrational frequencies are 1615, 3694, and 3802 cm-. The ground electronic
state is nondegenerate. (Note: the Avogadro's constant NA = 6.022 × 1023 mol-1).
Express your answer to three significant figures.
Ην ΑΣφ
qtotal =
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY