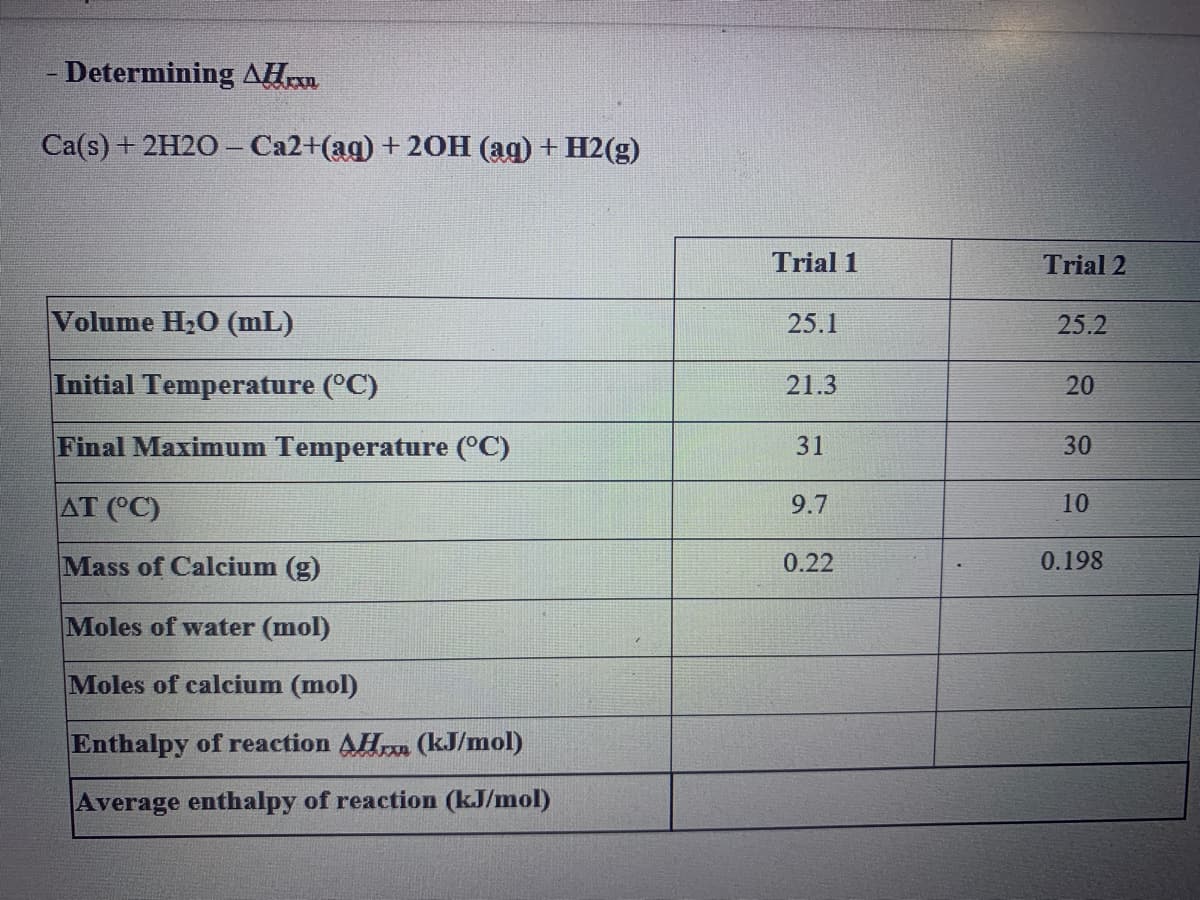
- Determining AH Ca('s) + 2H20-Са2+(ад) + 20Н (ад) + Н2(g) Trial 1 Trial 2 Volume H20 (mL) 25.1 25.2 Initial Temperature (°C) 21.3 20 Final Maximum Temperature (°C) 31 30 AT (°C) 9.7 10 Mass of Calcium (g) 0.22 0.198 Moles of water (mol) Moles of calcium (mol) Enthalpy of reaction AH (kJ/mol) Average enthalpy of reaction (kJ/mol)
Q: Ignition wires heat sample Water Stirrer Thermometer Insulated Sample Burning Steel outside dish…
A: Given the mass of isopentathalic acid, C8H6O4 taken = 0.7264 g Since the temperature of the water…
Q: Parts 1 and 2 Burning a Checto and Peanut Checto Peanut Peanut Cheeto 22.9°C Typc of Food Tested 1.…
A: The experiment data given is,
Q: Butane (CAH10) undergoes combustion in excess oxygen to generate gaseous carbon dioxide and water.…
A: Enthalpy of combustion: It is the amount of energy released when 1 mole of the reactant will burn in…
Q: Initial mass of food sample (g) 1.93 Final mass of food sample (g) 0.4: Initial temperature of water…
A: Given: Mass of Water =113.65g The initial temperature of water= 22.00 oC The final temperature of…
Q: Find the heat of reaction of: Mg(s) + 2H+(aq) --> H2(g) + Mg2+(aq) Given the following Rection…
A: Given : Volume of HCl solution = 100 mL = 0.100 L (since…
Q: Whenever organic matter is decomposed under oxygen-free(anaerobic) conditions, methane is one of the…
A: a) Concept introduction: Combustion is basically the reaction of a substance with oxygen Methane…
Q: the probability that a mole of perfume molecules expand from their open 100 ml diffuser into an…
A: Given, Volume of molecule initially = 100 ml Volume of molecule after expansion = 1L = 1000 ml…
Q: What mass of oxygen is consumed when 285.5 kJ of energy is evolved from the combustion of a mixture…
A: Consider the given balanced reaction as; H2 g + 12 O2 g → H2O l…
Q: Mass of calorimeter + water is 52.0753g, mass of water is 44.34g, mass of Unknown Solid is 5.00g,…
A: Total mass, (m) = (52.0753+5) g = 57.0753 g Change in temperature,…
Q: Nitromethane (CH3NO2) burns in air to produce significant amounts of heat. 2CH3NO2 (1) +…
A: The reaction is given below.
Q: O app.101edu.co E Apps MYUSU Outlook Canvas Labflow OneNote ov Chemt01 Tophat u USU Tutoring…
A:
Q: 1. What volume of methane, CH4 (g), measured at 25 °C and 745 torr must be burned in excess oxygen…
A:
Q: A bomb calorimeter, or a constant volume calorimeter, is a device often used to determine the heat…
A: Formula used : Qbomb = mH2O CpH2O (T2-T1) + Cbomb (T2-T1)
Q: (1) In class, we discussed state variables and their units. Show that 1 L-atm = 101.3 J.
A: Since, you have asked multiple question, as per our company guidelines we are supposed to answer the…
Q: m cold Mass of cold water (g) = 50 g Tic Initial temperature of cold water =15 C m hot Mass of hot…
A: Given: The mass of cold water and the mass of hot water is 50 g. The initial temperature of cold…
Q: Please fill in the following data tables with the provided experimental data. Part I Run 2: Boiling…
A: In this question, experimental data of calorimetry is given for two trials.
Q: What will be the final temperature of a mixture made from 25.0 g of water at 15.08C, 45.0 g of water…
A: When two or more substances(liquids in this situation) at different temperatures are mixed, then…
Q: 2AF(g)+ 0,(g)→ A,0(g) + F0(g) 2AF,(g)+20,(g)→ Cl,0(g) +3F,0(g) 2F,(g) + 0,(9)-2F,0(g) AH = 167.4kJ…
A: Interpretation - To determine the enthalpy change (∆H ) for the final reaction and also draw…
Q: Table 3 Mass (g) Calorimeter 19.78 Calorimeter & Room Temperature Water 117.97 Room Temperature…
A: The heat capacity of calorimeter is needed to be calculated.
Q: Part A Determine the mass of CO2 produced by burning enough of methane to produce 2.00 x 102 kJ of…
A: Number of moles of CH4 = Heat evolved/Heat of combustion Mass = number of moles x molar mass
Q: Copper (II) sulfate is a fungicide widely used in agriculture and gardening. In one of the…
A: Given: Compound is CuSO4.xH2O. And %(w/w) of water in compound = 36.40 %. Molar mass of H2O = Atomic…
Q: ANALYSIS Calculations: A1. (Refer back to Figure 3 for help with these calculations) The following…
A: Solution specific heat of a solid or liquid is that the quantity of warmth that raises the…
Q: In an experiment, a 0.4486 g sample of 1,9-nonanediol (C9H20O2) is burned completely in a bomb…
A:
Q: Acetylene burns in air according to the following equation. C2H2(g) + 5/2 02(g) → 2 CO2(g) + H20(g)…
A:
Q: Mixing 10.0 g of CaC2 and 25.0 mL of H2O (p = 0.997044 g/mL) conforms to the thermochemical equation…
A: Here we are required to find the amount of heat evolved when 10 gm of calcium carbide is mixed with…
Q: certain mass of substance 10 releases 3.19 kJ of energy. If the initial temperature was 91.8 and…
A: q = mc∆T q is the heat ,m is the mass, c is the specific heat, ∆T = Change in temperature q =…
Q: Complete the table by computing for the unknown parameter. [H+] [OH–] pH pOH 1…
A:
Q: Potassium hydroxide pellets (KOH) are placed in 100.00 mL of water. The mass of KOH used is 40.005…
A: Volume of water = 100.0 mL Mass of KOH = 40.005 g Enthalpy of solution ∆ H = - 57.6 kJ/mol Initial…
Q: Consider the following combustion reaction for acetone, C3H6O (1) + 4 O2 (g) → 3 CO2 (g) + 3 H2O (g)…
A:
Q: Consider the reaction. heat 2 Al(s) + Fe,0,(s) – Al,0,(s) + 2 Fe(l) If 29.9 kg Al reacts with an…
A: Mole is the amount of the substance that contains the same number of particles or atoms or…
Q: a) Mass of calorimeter 5.0 g b) Mass of calorimeter plus water 34.1 g c) Mass of ammonium chloride…
A: The data given is,
Q: A large sport utility vehicle has a mass of 2500 kg. Calculate the mass of CO2 emitted into the…
A:
Q: Mass of cold water т cold т (g) = 50 g Tic Initial temperature of cold water =15 C m hot Mass of hot…
A: We have given that Mass of cold water mcold = 50 g & Initial temperature of cold water Ti (cold)…
Q: Mass of empty Calorimeter is 7.73g, mass of calorimeter + water is 46.875g, mass of water is…
A: Given the initial temperature of metal, Ti = 100 oC When the hot metal is put inside the…
Q: A student runs two experiments with a constant-volume "bomb" calorimeter containing 1300. g of water…
A: Heat Of Combustion of Benzoic Acid = 26.454 kJ/g = 26454 J/gMass of Benzoic Acid = 6 gHeat released…
Q: Consider these reactions: AH = -184.6 kJ Reaction 1: H₂(g) + Cl₂ (g) → 2HCl(g) Reaction 2:20F2 (g)…
A:
Q: Normal text Times New. - 12 BIUA + E- 1E = E- E - E E X 2 3 I c. Water from the sodium hydroxide…
A:
Q: N2(g)+2O2(g)⟶2NO2(g)ΔH=+66.4 kJ Use Reaction 3. How much energy (in kJ) is absorbed when…
A: Recall the reaction, N2 + 2O2 ------> 2NO2 ∆H = +66.4 KJ Volume of NO2=159 L Energy absorbed =…
Q: Be sure to answer all parts. Isooctane (CgH1s; d= 0.692 g/mL) is used as the fuel during a test of a…
A:
Q: A student runs two experiments with a constant-volume "bomb" calorimeter containing 1400. g of water…
A: Mass of water = 1400 g Mass of benzoic acid = 7.000 g Mass of ethane = 5.790 g Given balanced…
Q: The combustion of sucrose (C12H22011) proceeds according to the following reaction. What is the…
A: Energy change of a reaction is represents by ∆H , it is also called enthalpy change. If energy is…
Q: 10. Ethanol OH weighing 3.56 g is burned in a calorimeter. The temperature rises from 15.C to C. The…
A: During the burning of ethanol the heat released is absorbed by water and a calorimeter. First of…
Q: Consider these reactions: Reaction 1: H2 (9) + Cl2 (g) → 2HC1(g) AH = –184.6 kJ Reaction 2: 20F2 (g)…
A: Given that, H2(g) + Cl2(g)------->2HCl(g) ∆H=-184.6kJ Calculate mass of chlorine gas to…
Q: When ignited, solid ammonium dichromate decomposes in a fiery display. This is the reaction for a…
A: To calculate change in entropy of universe, we need entropy change of system and surrounding.
Q: How much energy is required to raise the temperature of 12.2 grams of gaseous nitrogen from 23.0 °C…
A: In the given question, the energy required to raise the temperature of 0.368 kg of copper from 23.0…
Q: Original temperature of HCI is 20.6 degrees Celsius, Original temperature of NaOH is 20.6 degrees…
A: Given data, Initial temperature of HCl = 20.6oC Initial temperature of NaOH = 20.6oC Final…
Q: m cold Mass of cold water (g) = 50 g %3D Tic Initial temperature of cold water =15 C m hot Mass of…
A: When the hot and cold water mixed together then the intermediate temperature is the final…
Q: Be sure to answer all parts. Determine the amount of heat (in kJ) associated with the production of…
A:
Q: Ex 1) When a piece of zinc is dipped in an aqueous solution of hydrochloric acid it reacts with the…
A: Since you have asked a question with multiple subparts, we will answer only first three subparts. In…
Step by step
Solved in 4 steps with 4 images

- Use only the first decimal points (X.X) for atomic masses. R = 8.314 L*kPa/mole*K . In a reactor at 200.0 kPA and 401 L, hydrogen and oxygen combine by the following reaction:2H2(g) + O2(g) --> 2H2O(g)51.2 g of hydrogen and 392 g of oxygen are placed into the reactor. What is the limiting reagent? H2 or O2 What is the final temperature of the system in degree celsius?Use only the first decimal points (X.X) for atomic masses. R = 8.314 L*kPa/mole*K . In a reactor at 200.0 kPA and 401 L, hydrogen and oxygen combine by the following reaction:2H2(g) + O2(g) --> 2H2O(g)51.2 g of hydrogen and 392 g of oxygen are placed into the reactor. What is the limiting reagent? H2 or O2 What is the final temperature of the system?A mixture of Al2O3(s) and CuO(s) weighing 18.371 mg was heated under H2(g) at 1 0008C to give 17.462 mg of Al2O3(s) 1 Cu(s). The other product is H2O(g). Find wt% Al2O3 in the original mixture.
- Acetaminophen, a popular drug taken as pain reliever and fever reducer, is produced together with acetic acid from the reaction of 3.05 g 4-aminophenol and 4.1 ml of acetic anhydride. Acetaminophen was extracted at 60% yield. Density of acetic anhydride at 20 C, 1.08 g/ml. Calculate the actual no. of grams of acetaminophen produced. [Determine L.R., E.R.]Ozone is a trace atmospheric gas which plays an important role in screening the Earth from harmful ultraviolet radiation, and the abundance of ozone is commonly reported in Dobson units. Imagine a column passing up through the atmosphere. The total amount of O3 in the column divided by its cross-sectional area is reported in Dobson units with 1 Du = 0.4462 mmol m−2. What amount of O3 (in moles) is found in a column of atmosphere with a cross-sectional area of 1.00 dm2 if the abundance is 250 Dobson units (a typical midlatitude value)? In the seasonal Antarctic ozone hole, the column abundance drops below 100 Dobson units; how many moles of O3 are found in such a column of air above a 1.00 dm2 area? Most atmospheric ozone is found between 10 and 50 km above the surface of the Earth. If that ozone is spread uniformly through this portion of the atmosphere, what is the average molar concentration corresponding to (a) 250 Dobson units, (b) 100 Dobson units?How would each of the following errors affect the determination of the molar mass of the unknown (Increase/Decrease/No effect)? a. Thermometer reads 2.0o higher than the true temperature. b. Some of the t-butanol was unknowingly spilled after it had been weighed but before the solute was added. 3. A student accidentally added acetylsalicylic acid (MW = 180.157 g/mol) rather than salicylic acid (MW = 138.121 g/mol), if no other mistakes were made, how would this error affect the determined molal freezing point depression constant for t-butyl alcohol (Kf)? 4. Based on the Tf and Kf you determined for t-butyl alcohol, predict the freezing point of a t-butyl alcohol solution containing 0.530 m NaCl. Assume 1.9 is the van’t Hoff factor for NaCl in t-butyl alcohol. Show your work.
- A student performed the experiment described in this module, using 7.00 mL of a 1.8% H2O2 solution with a density of 1.01 g mL-1. The water temperature was 20 ° C, and the barometric pressure in the laboratory was 30.02 in. Hg. After the student immersed the yeast in the peroxide solution, she collected 45.1 mL of O2. (11) Calculate the percent error for the experiment.A student performed the experiment described in this module, using 7.00 mL of a 1.8% H2O2 solution with a density of 1.01 g mL-1. The water temperature was 20 ° C, and the barometric pressure in the laboratory was 30.02 in. Hg. After the student immersed the yeast in the peroxide solution, she collected 45.1 mL of O2. (11) Calculate the percent error for the experiment. What is the observed molar volume?The volume of HCI is 100 mL , mass of solid added is 1.008g, moles of solid 0.0276 Mol, mass of HCI is 100g, initial temperature is 21.8 degrees Celsius, Final temperature is 30.7 degrees Celsius, Calculate q rxn(= -mc Delta T of HCI),
- Calculations for experimentally determining R Trial 1 Trial 2 Mass of Mg ribbon (g) 0.031 0.039 Temperature of H2(g) (°C) 24.0 24.0 Volume of H2 collected (mL) 31.80 40.10 Atmospheric pressure (torr) 754 754 Vapor pressure of water (torr) Volume of H2 gas in liters (L) Temperature of H2 gas in Kelvin (K) Moles of H2 gas (mol) Pressure of H2 gas in atmospheres (atm) Experimental value of R (L·atm/(mol·K)) Average value of R (L·atm/(mol·K)): Percent error (%)Use 0.08206 L·atm/(mol·K) for the theoretical value of R: The hydrogen generated in this lab was a product of the reaction between magnesium and hydrochloric acid. Which of these reactants is the limiting reactant?: Magnesium or Hydrochloric Acid? Explain reasonsing.Report sheet analysis of KCIO3/KCI Unknown substance 1 Mass of empty 400mL beaker 164.93g, Mass of 30mL beaker+test tube 96.181g, Mass of 30mL beaker+test tube+unkown mixture 97.218g, Mass of unkown substance 1 1.037g, Mass of 400mL beaker and water 304.97g, Temperature of O2 20 celsius, Atmospheric pressure 750.1 torr. Mass of water ___, Volume of O2___, Vapor pressure of water ___torr, Partial pressure of O2 (Po2) ___torr, Partial pressure of O2 (Po2) ___atm, Moles of O2 (no2) ___, Moles of KCIO3 in unkown ___, Grams of KCIO3 in known ___, % by mass KCIO3 in unknown ___.Find the mass of hydrogen gas produced by the reaction of 4.0g Al with excess sulfuric acid by using dimensional analysis. 2Al+3H2SO4-> Al2(SO4)3 +3H2

