Please fill in the following data tables with the provided experimental data. Part I Run 2: Boiling water 184.29 g Run 1: Ice-water Mass of empty beaker 184.29 g Mass of beaker plus pennies 201.35 g 201.35 g Volume of water in calorimeter 28.3 mL 28.3 mL O degree celsius Initial Temperature of pennies (assume same as water bath) Initial Temperature of water in 100 degree Celsius 21.3 degree celsius 21.3 degree celsius calorimeter 20.1 degree celsius 24.6 degree celsius Final Temperature of water and pennies in calorimeter
Please fill in the following data tables with the provided experimental data. Part I Run 2: Boiling water 184.29 g Run 1: Ice-water Mass of empty beaker 184.29 g Mass of beaker plus pennies 201.35 g 201.35 g Volume of water in calorimeter 28.3 mL 28.3 mL O degree celsius Initial Temperature of pennies (assume same as water bath) Initial Temperature of water in 100 degree Celsius 21.3 degree celsius 21.3 degree celsius calorimeter 20.1 degree celsius 24.6 degree celsius Final Temperature of water and pennies in calorimeter
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 58P
Related questions
Question
100%
Please answer
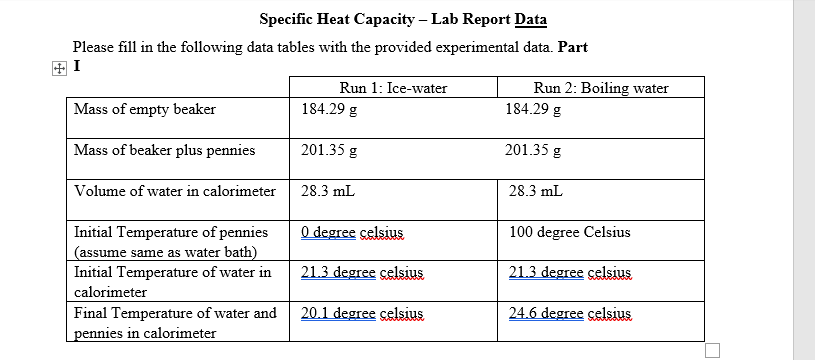
Transcribed Image Text:Specific Heat Capacity – Lab Report Data
Please fill in the following data tables with the provided experimental data. Part
I
Run 1: Ice-water
Run 2: Boiling water
184.29 g
Mass of empty beaker
184.29 g
Mass of beaker plus pennies
201.35 g
201.35 g
Volume of water in calorimeter
28.3 mL
28.3 mL
O degree celsius
Initial Temperature of pennies
(assume same as water bath)
Initial Temperature of water in
100 degree Celsius
21.3 degree celsius
21.3 degree celsius
calorimeter
Final Temperature of water and
20.1 degree celsius
24.6 degree celsius
pennies in calorimeter
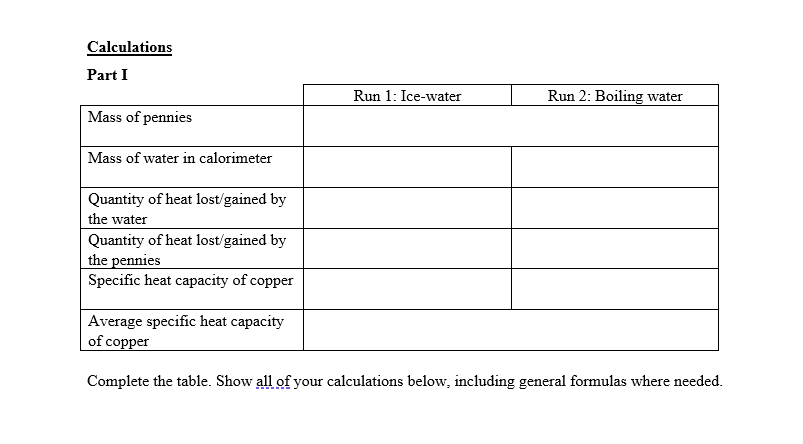
Transcribed Image Text:Calculations
Part I
Run 1: Ice-water
Run 2: Boiling water
Mass of pennies
Mass of water in calorimeter
Quantity of heat lost/gained by
the water
Quantity of heat lost/gained by
the pennies
Specific heat capacity of copper
Average specific heat capacity
of copper
Complete the table. Show all of your calculations below, including general formulas where needed.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning