Nitromethane (CH3NO2) burns in air to produce significant amounts of heat. 2CH3NO2 (1) + 3/202(g)→2CO2(g)+3H2O(1) + N2(g) AH&m = -1418 kJ How much heat is produced by the complete reaction of 5.29 kg of nitromethane? Express your answer to three significant figures and include the appropriate units
Nitromethane (CH3NO2) burns in air to produce significant amounts of heat. 2CH3NO2 (1) + 3/202(g)→2CO2(g)+3H2O(1) + N2(g) AH&m = -1418 kJ How much heat is produced by the complete reaction of 5.29 kg of nitromethane? Express your answer to three significant figures and include the appropriate units
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter10: Energy
Section: Chapter Questions
Problem 38QAP: In the “Chemistry in Focus” segment Firewalking: Magic or Science?, it is claimed that one reason...
Related questions
Question
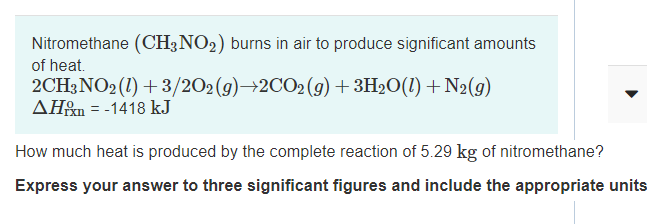
Transcribed Image Text:Nitromethane (CH3NO2) burns in air to produce significant amounts
of heat.
2CH3NO2 (1) + 3/202(g)→2CO2(g)+3H2O(1) + N2(g)
AH&m = -1418 kJ
How much heat is produced by the complete reaction of 5.29 kg of nitromethane?
Express your answer to three significant figures and include the appropriate units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning