Diethyl ether has a freezing point of -116.2 degrees C and a Kf of 1.79 degrees C/m. What is the freezing point of a 1.25 m solution of SO3 in diethyl ether?
Diethyl ether has a freezing point of -116.2 degrees C and a Kf of 1.79 degrees C/m. What is the freezing point of a 1.25 m solution of SO3 in diethyl ether?
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter14: Mixtures And Solutions
Section14.4: Colligative Properties Of Solutions
Problem 46PP
Related questions
Question
Diethyl ether has a freezing point of -116.2 degrees C and a Kf of 1.79 degrees C/m. What is the freezing point of a 1.25 m solution of SO3 in diethyl ether?
Expert Solution
Step 1
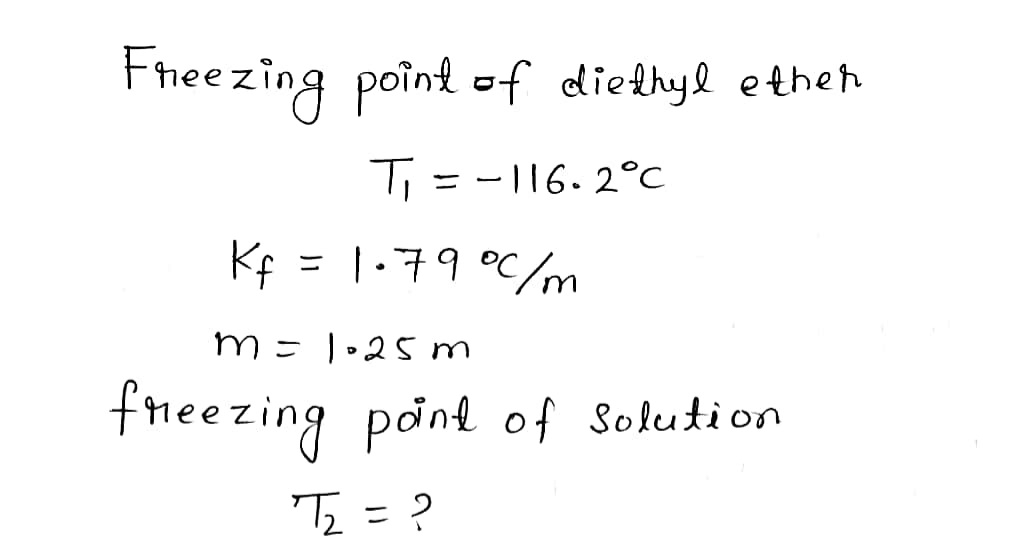
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
Expert Answers to Latest Homework Questions
Q: If a dc source supplied RL load throw step down chopper in fig below if K = 0.75 Imin (11) =4A, Vs =…
Q: If a dc source supplied RL load throw step down chopper in fig below if K = 0.75 Imin (11) =4A, Vs =…
Q: Typed please
Q: The quadratic Taylor series polynomial approximation at the point (1, 2) to the function
f(x,y) 2x +…
Q: What is the expected product of the following reaction?
:0:
H
:0:
:0:
::
LOH
:0:
:OH
10:
::
???
Q: None
Q: If the marginal propensity to save in an economy is 0.2, and the marginal
propensity to import is…
Q: None
Q: If the marginal propensity to save in an economy is 0.2, and the marginal
propensity to import is…
Q: The movie below shows some molecules in a tiny sample of a mixture of gases.
key
carbon
hydrogen…
Q: Pause and solve Last year Acme, Inc.
had $16.4 million in revenue, $1.2
million of operating…
Q: None
Q: Instruction:
1. Total acidity (expressed as % tartaric acid): To a 10-ml aliquot of the fermenting…
Q: None
Q: Find a cubic polynomial (that is, f(x) = ax³ + bx² + cx + d) that has horizontal tangents at the…
Q: A FCFS job Sequence with job work timė A;6- B2- C;8- D;3- E9.
Calculate the average number of jobs…
Q: Problem 7-40 (LO. 3, 5)
Mila purchased a Zaffre Corporation $100,000 bond 10 years ago for its face…
Q: Report Sheet Electrochemistry: Silver lon Equilibrium Part A A.
Determination of Equilibrium…
Q: Typed please
Q: The rate constant for this first-order reaction is 0.290 s¹ at 400 °C.
A
products
How long, in…
Q: A coil with a self-inductance of 9.6 H carries a current that varies with time according to I(t)…