Differential Distillation A 100 gmole liquid mixture containing 50 mole% n-heptane and 50 mole% n-octane at is to be subjected to a differential distillation at atmospheric pressure. The process is stopped when the mole fraction of MVC (n-heptane) in the still reaches 0.33. Determine the amount of composited distillate collected and its mole fraction a) The data given as: X00.012 0.039 0.097 0.157 0.284 0.312 0.487 0.510 0.655 y 0 0.025 0.078 0.184 0.279 0.459 0.492 0.674 0.698 0.810
Differential Distillation A 100 gmole liquid mixture containing 50 mole% n-heptane and 50 mole% n-octane at is to be subjected to a differential distillation at atmospheric pressure. The process is stopped when the mole fraction of MVC (n-heptane) in the still reaches 0.33. Determine the amount of composited distillate collected and its mole fraction a) The data given as: X00.012 0.039 0.097 0.157 0.284 0.312 0.487 0.510 0.655 y 0 0.025 0.078 0.184 0.279 0.459 0.492 0.674 0.698 0.810
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.20QAP
Related questions
Question
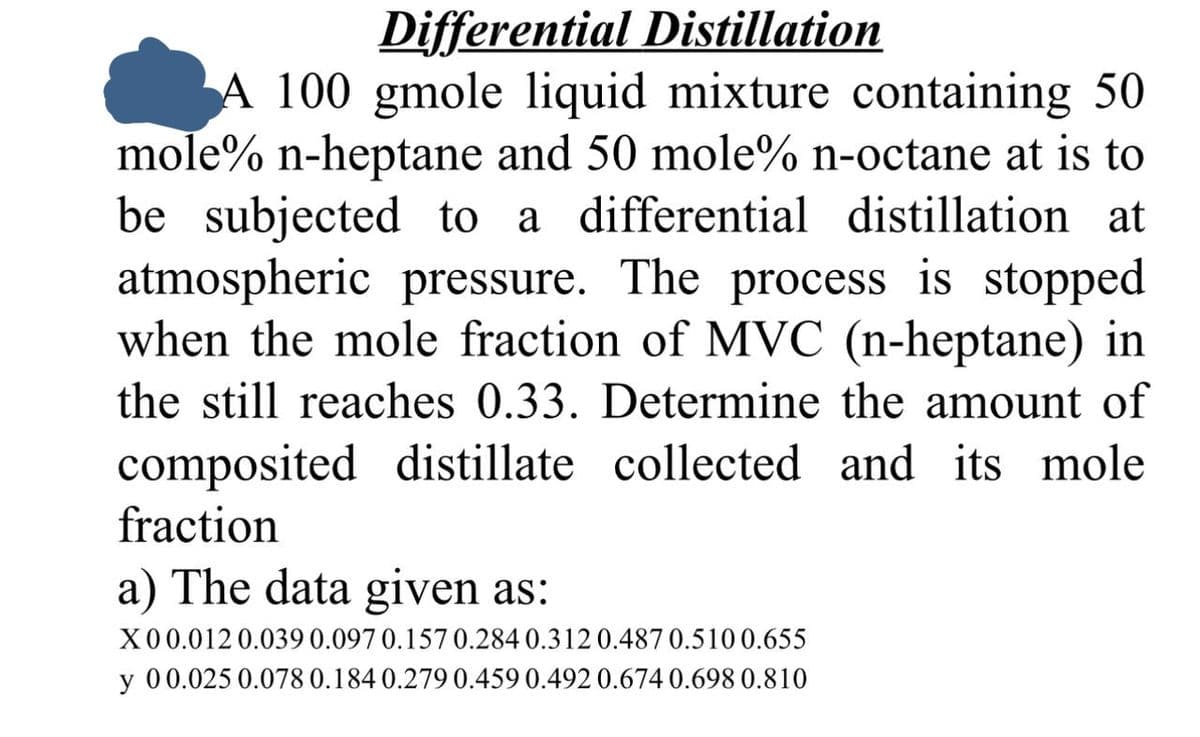
Transcribed Image Text:Differential Distillation
A 100 gmole liquid mixture containing 50
mole% n-heptane and 50 mole% n-octane at is to
be subjected to a differential distillation at
atmospheric pressure. The process is stopped
when the mole fraction of MVC (n-heptane) in
the still reaches 0.33. Determine the amount of
composited distillate collected and its mole
fraction
a) The data given as:
X00.012 0.039 0.097 0.157 0.284 0.312 0.487 0.510 0.655
y 0 0.025 0.078 0.184 0.279 0.459 0.492 0.674 0.698 0.810
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

