a.) A 12 kmol liquid mixture with XSi = 0.8, xca = 0.2 at 1500oC was cooled to 1030oC. Determine the amount of liquid and pure solid Si that are in equilibrium with each other. b.) Suppose the mixture in (a) is cooled further to 900oC. how many kg of CaSi2 is formed? (Molar weights: Ca – 40 g/mol, Si – 28 g/mol) c.)At xSi = 0.694 and T = 1030oC, is the system solid, liquid, or both?
a.) A 12 kmol liquid mixture with XSi = 0.8, xca = 0.2 at 1500oC was cooled to 1030oC. Determine the amount of liquid and pure solid Si that are in equilibrium with each other. b.) Suppose the mixture in (a) is cooled further to 900oC. how many kg of CaSi2 is formed? (Molar weights: Ca – 40 g/mol, Si – 28 g/mol) c.)At xSi = 0.694 and T = 1030oC, is the system solid, liquid, or both?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter7: Equilibria In Multiple-component Systems
Section: Chapter Questions
Problem 7.57E
Related questions
Question
a.) A 12 kmol liquid mixture with XSi = 0.8, xca = 0.2 at 1500oC was cooled to 1030oC. Determine the amount of liquid and pure solid Si that are in equilibrium with each other.
b.) Suppose the mixture in (a) is cooled further to 900oC. how many kg of CaSi2 is formed? (Molar weights: Ca – 40 g/mol, Si – 28 g/mol)
c.)At xSi = 0.694 and T = 1030oC, is the system solid, liquid, or both?
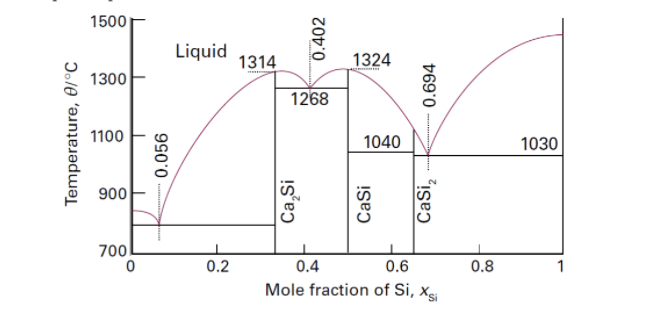
Transcribed Image Text:1500
Liquid
1314
1324
1300
1268
1100
1040
1030
900
700
0.2
0.4
0.6
0.8
1
Mole fraction of Si, x
Temperature, 01°C
0.056
Ca,Si
0.402
CaSi
CaSi,
0.694
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
