Dilute phosphoric acid(K1 = 7.5 x 10-3) solution is titrated with standard NaOH solution to form NaH,PO4. Which pH indicators below could NOT be used the detect the end-point? 7. 10 Methyl Orange Bromeresol Green Methyl Red Bromthymol Blue Phenolphthalein A. methyl orange and bromocresol green B. bromocresol green and methyl red C. methyl red and bromothymol blue D. bromothymol blue and phenolphthalein
Dilute phosphoric acid(K1 = 7.5 x 10-3) solution is titrated with standard NaOH solution to form NaH,PO4. Which pH indicators below could NOT be used the detect the end-point? 7. 10 Methyl Orange Bromeresol Green Methyl Red Bromthymol Blue Phenolphthalein A. methyl orange and bromocresol green B. bromocresol green and methyl red C. methyl red and bromothymol blue D. bromothymol blue and phenolphthalein
Chapter14: Principles Of Neutralization Titrations
Section: Chapter Questions
Problem 14.9QAP
Related questions
Question
100%
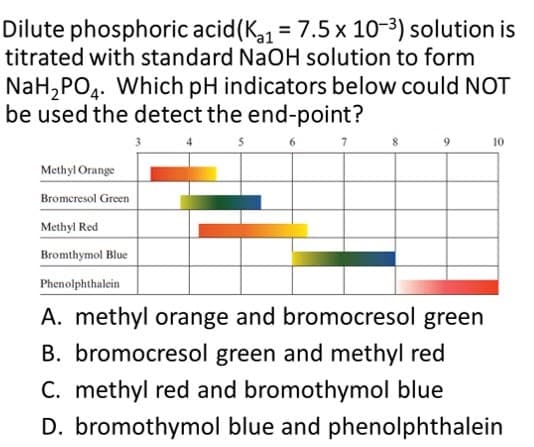
Transcribed Image Text:Dilute phosphoric acid(K = 7.5 x 10-3) solution is
titrated with standard NaOH solution to form
NaH,PO4. Which pH indicators below could NOT
be used the detect the end-point?
10
Methyl Orange
Bromeresol Green
Methyl Red
Bromthymol Blue
Phenolphthalein
A. methyl orange and bromocresol green
B. bromocresol green and methyl red
C. methyl red and bromothymol blue
D. bromothymol blue and phenolphthalein
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning