Dilute the stock solution: Using the solutions in the burets, carefully measure the following quantities of Co(NO3)2 solution and water into 5 large test tubes, to a total of 5.00 mL in each test tube. Yes, the optional video says to make 10.00 mL total, but 5.00 mL is sufficient at this time and generates less waste. Use the buret to measure the cobalt solution and the water. Test tube No. 1 2 3 4 5 mL Co2+ Soln 1.00 2.00 3.00 4.00 5.00 mL H2O 4.00 3.00 2.00 1.00 0.00 Total mL 5.00 5.00 5.00 5.00 5.00 Mix each test tube thoroughly: hold the top of the tube and mix the bottom well. Transfer a portion of each diluted sample into a clean, dry sample cuvette. fill the concentration table and do calculations please
Dilute the stock solution: Using the solutions in the burets, carefully measure the following quantities of Co(NO3)2 solution and water into 5 large test tubes, to a total of 5.00 mL in each test tube. Yes, the optional video says to make 10.00 mL total, but 5.00 mL is sufficient at this time and generates less waste. Use the buret to measure the cobalt solution and the water. Test tube No. 1 2 3 4 5 mL Co2+ Soln 1.00 2.00 3.00 4.00 5.00 mL H2O 4.00 3.00 2.00 1.00 0.00 Total mL 5.00 5.00 5.00 5.00 5.00 Mix each test tube thoroughly: hold the top of the tube and mix the bottom well. Transfer a portion of each diluted sample into a clean, dry sample cuvette. fill the concentration table and do calculations please
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 109AE: Patients undergoing an upper gastrointestinal tract laboratory test are typically given an X-ray...
Related questions
Question
Dilute the stock solution:
- Using the solutions in the burets, carefully measure the following quantities of Co(NO3)2 solution and water into 5 large test tubes, to a total of 5.00 mL in each test tube. Yes, the optional video says to make 10.00 mL total, but 5.00 mL is sufficient at this time and generates less waste. Use the buret to measure the cobalt solution and the water.
|
Test tube No. |
1 |
2 |
3 |
4 |
5 |
|
mL Co2+ Soln |
1.00 |
2.00 |
3.00 |
4.00 |
5.00 |
|
mL H2O |
4.00 |
3.00 |
2.00 |
1.00 |
0.00 |
|
Total mL |
5.00 |
5.00 |
5.00 |
5.00 |
5.00 |
- Mix each test tube thoroughly: hold the top of the tube and mix the bottom well.
- Transfer a portion of each diluted sample into a clean, dry sample cuvette.
fill the concentration table and do calculations please
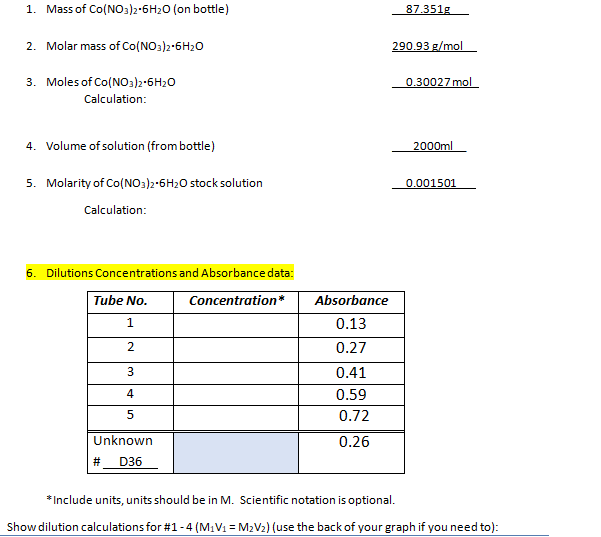
Transcribed Image Text:1. Mass of Co(NO3)2-6H₂O (on bottle)
2. Molar mass of Co(NO3)2-6H₂O
3. Moles of Co(NO3)2-6H₂O
Calculation:
4. Volume of solution (from bottle)
5. Molarity of Co(NO3)2-6H₂O stock solution
Calculation:
6. Dilutions Concentrations and Absorbance data:
Tube No.
Concentration*
1
2
3
4
5
Unknown
# D36
Absorbance
0.13
0.27
0.41
0.59
0.72
0.26
87.351g
290.93 g/mol
0.30027 mol
2000ml
0.001501
*Include units, units should be in M. Scientific notation is optional.
Show dilution calculations for #1 -4 (M₁V₁ = M₂V₂) (use the back of your graph if you need to):
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

