Din Diarin wants to methylate the diphenol shown below using methyl bromide. The reaction is a simple nucleophilic substitution, and requires both phenol groups to be deprotonated by a base so that the resulting phenoxide may attack the alkyl halide and form a C-O bond. но. Base + 2 Br DMF/H20 ОН NH2 Potential bases 1) КОН 3) K2CO3 4) CSOH 5) a) Which of the potential base(s) would likely be an acceptable choice to ensure an efficient reaction under the current reaction conditions? Briefly explain your answer.
Din Diarin wants to methylate the diphenol shown below using methyl bromide. The reaction is a simple nucleophilic substitution, and requires both phenol groups to be deprotonated by a base so that the resulting phenoxide may attack the alkyl halide and form a C-O bond. но. Base + 2 Br DMF/H20 ОН NH2 Potential bases 1) КОН 3) K2CO3 4) CSOH 5) a) Which of the potential base(s) would likely be an acceptable choice to ensure an efficient reaction under the current reaction conditions? Briefly explain your answer.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter14: Elimination
Section: Chapter Questions
Problem 35CTQ
Related questions
Question
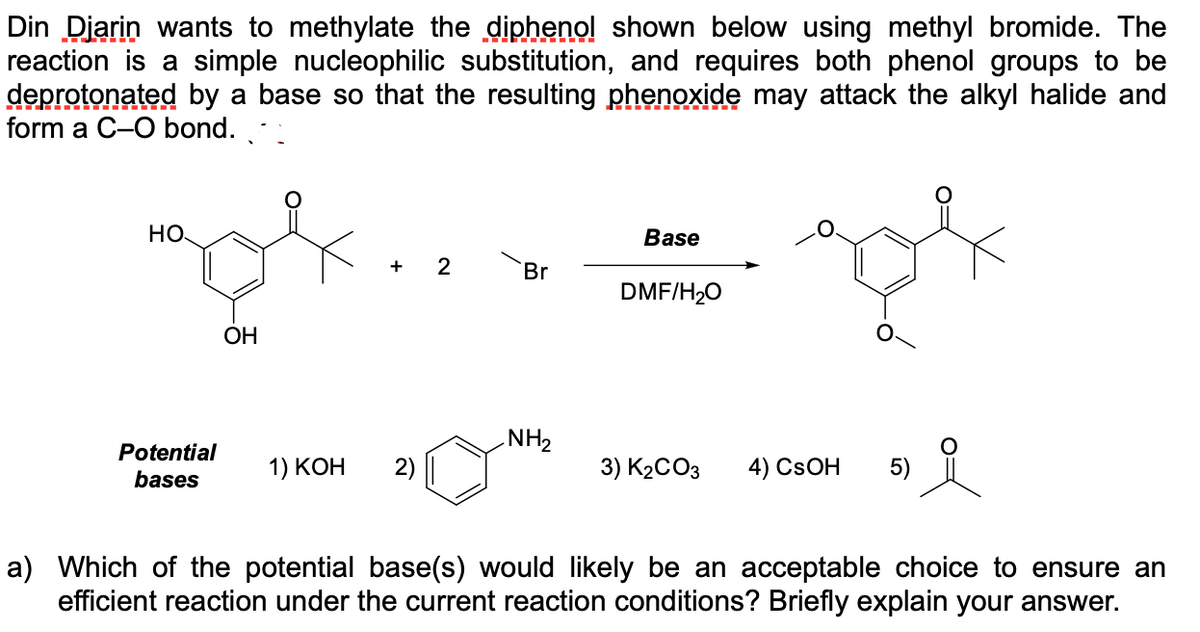
Transcribed Image Text:Din Diarin wants to methylate the diphenol shown below using methyl bromide. The
reaction is a simple nucleophilic substitution, and requires both phenol groups to be
deprotonated by a base so that the resulting phenoxide may attack the alkyl halide and
form a C-O bond.
HO
Base
+
Br
DMF/H20
ОН
NH2
Potential
bases
1) КОН
2)
3) K2CO3
4) CSOH
5)
a) Which of the potential base(s) would likely be an acceptable choice to ensure an
efficient reaction under the current reaction conditions? Briefly explain your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning