Direction: Write (v) if the statement is correct and (X) if the statement is incorrect. 1. The heat lost by one object equals the heat gained by another object. 2. Temperature indicates the direction in which heat flows while heat is the actual energy transferred. 3. Heat is the energy that transfer from a body of higher temperature to another body of lower temperature. _4. Heat is the quantity of kinetic energy absorbed or given off by a body. 5. Waste heat is the amount of heat that is not converted to into work. _6. Heat can increase the internal energy of an object as it excites the molecules when subjected to lower temperatures. _7. In measuring body temperature when you have a fever, the heat is flowing from the thermometer to the armpit. 8. Hot objects have high internal energy as the molecules in them are slow moving. _9. Energy transfer in the form of heat can result in the performance of work upon the system or the surroundings. 10. Absolute zero (OK) corresponds to -273° C.
Direction: Write (v) if the statement is correct and (X) if the statement is incorrect. 1. The heat lost by one object equals the heat gained by another object. 2. Temperature indicates the direction in which heat flows while heat is the actual energy transferred. 3. Heat is the energy that transfer from a body of higher temperature to another body of lower temperature. _4. Heat is the quantity of kinetic energy absorbed or given off by a body. 5. Waste heat is the amount of heat that is not converted to into work. _6. Heat can increase the internal energy of an object as it excites the molecules when subjected to lower temperatures. _7. In measuring body temperature when you have a fever, the heat is flowing from the thermometer to the armpit. 8. Hot objects have high internal energy as the molecules in them are slow moving. _9. Energy transfer in the form of heat can result in the performance of work upon the system or the surroundings. 10. Absolute zero (OK) corresponds to -273° C.
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter14: Heat And Heat Transfer Methods
Section: Chapter Questions
Problem 53PE: A person inhales and exhales 2.00 L of 37.0C air, evaporating 4.00102g of water from the lungs and...
Related questions
Question
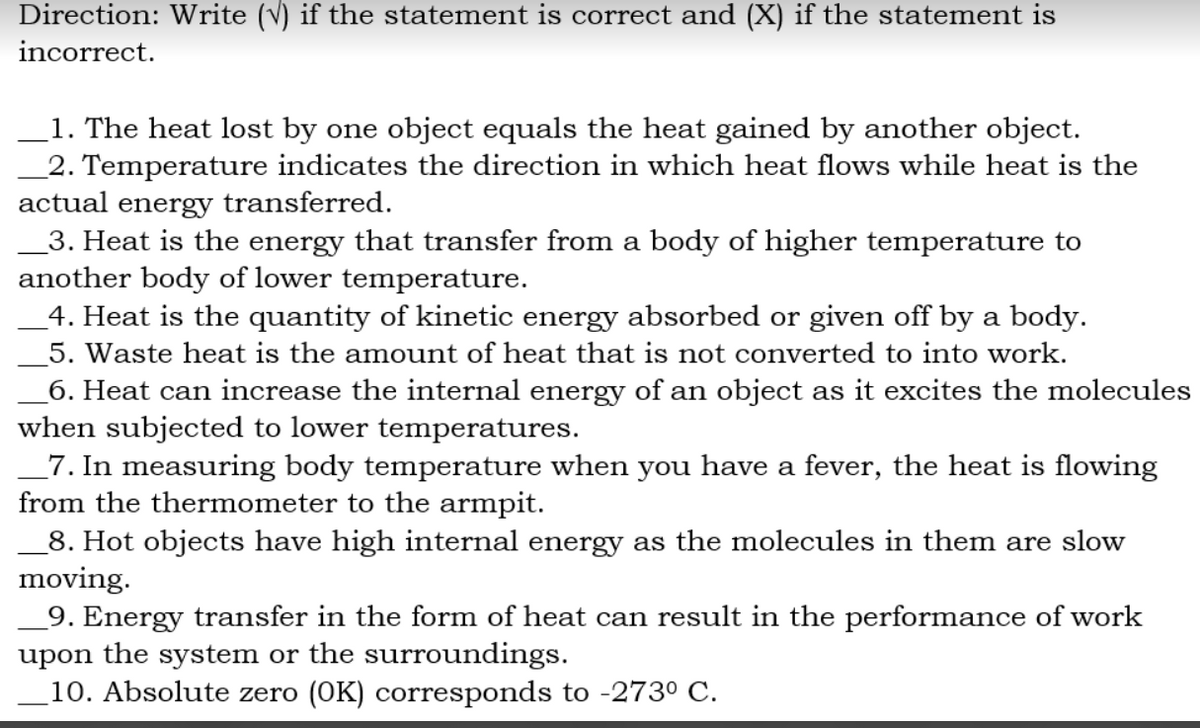
Transcribed Image Text:Direction: Write (V) if the statement is correct and (X) if the statement is
incorrect.
1. The heat lost by one object equals the heat gained by another object.
2. Temperature indicates the direction in which heat flows while heat is the
actual energy transferred.
_3. Heat is the energy that transfer from a body of higher temperature to
another body of lower temperature.
4. Heat is the quantity of kinetic energy absorbed or given off by a body.
5. Waste heat is the amount of heat that is not converted to into work.
6. Heat can increase the internal energy of an object as it excites the molecules
when subjected to lower temperatures.
_7. In measuring body temperature when you have a fever, the heat is flowing
from the thermometer to the armpit.
8. Hot objects have high internal energy as the molecules in them are slow
moving.
_9. Energy transfer in the form of heat can result in the performance of work
upon the system or the surroundings.
10. Absolute zero (OK) corresponds to -2730 C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning