Directions CHCЬ CH3CI CCla Write number of valence electrons for cach atom and find the total number of valence electrons Determine center atom and draw the skeleton Write the full Lewis Structure with all valence electrons Determine non-zero formal charges Center atom has regions Name the electronic geometry Center atom: # bonding atoms? # lone pairs? Name the molecular gcometry Draw the molecular geometry Identify polar covalent pairs. & Add dipole arrows for each polar bond Is there a net dipole? Is the molecule Polar or Nonpolar?
Directions CHCЬ CH3CI CCla Write number of valence electrons for cach atom and find the total number of valence electrons Determine center atom and draw the skeleton Write the full Lewis Structure with all valence electrons Determine non-zero formal charges Center atom has regions Name the electronic geometry Center atom: # bonding atoms? # lone pairs? Name the molecular gcometry Draw the molecular geometry Identify polar covalent pairs. & Add dipole arrows for each polar bond Is there a net dipole? Is the molecule Polar or Nonpolar?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter4: Molecular Structure And Orbitals
Section: Chapter Questions
Problem 123CP: Carbon monoxide (CO) forms bonds to a variety of metals and metal ions. liS ability to bond to iron...
Related questions
Question
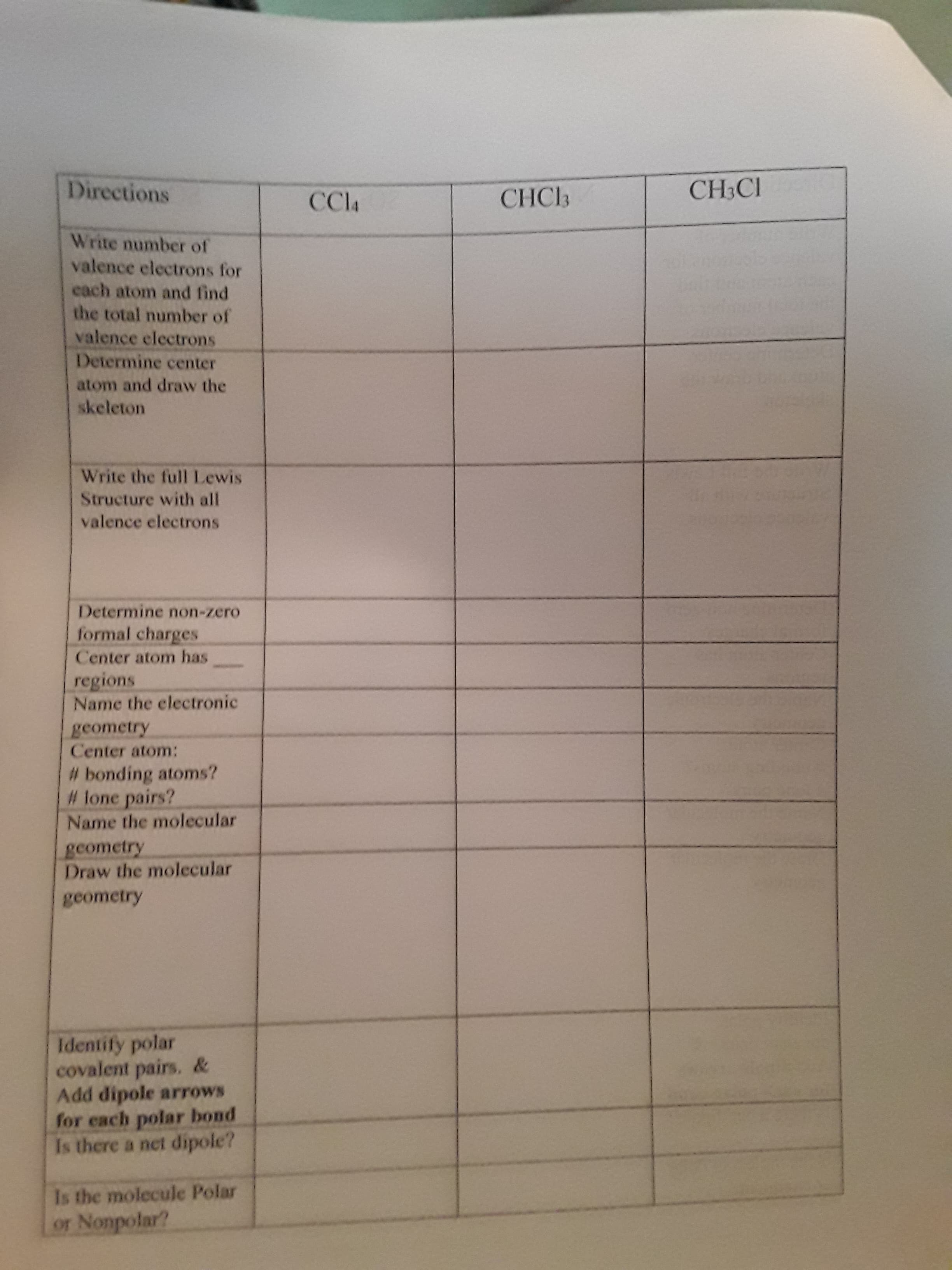
Transcribed Image Text:Directions
CHCЬ
CH3CI
CCla
Write number of
valence electrons for
cach atom and find
the total number of
valence electrons
Determine center
atom and draw the
skeleton
Write the full Lewis
Structure with all
valence electrons
Determine non-zero
formal charges
Center atom has
regions
Name the electronic
geometry
Center atom:
# bonding atoms?
# lone pairs?
Name the molecular
gcometry
Draw the molecular
geometry
Identify polar
covalent pairs. &
Add dipole arrows
for each polar bond
Is there a net dipole?
Is the molecule Polar
or Nonpolar?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 19 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning