Number of Unshared Electron Number of Bond Groups Around Central Atom Molecular Shape Pairs Around the Central Atom Linear Bent Trigonal Planar Trigonal Pyramidal Tetrahedral 2. What is the electrostatic charge of all electron groups, either shared (ie. bonds) or unshared? 3. Since all electron groups have this same charge, do they attract or repel each other? 4. How many unshared electron pairs are present in the carbon atom in CO,? 5. Considering your answers to the previous three questions, explain why CO, is not bent? 6. Explain why H,O is not linear.
Number of Unshared Electron Number of Bond Groups Around Central Atom Molecular Shape Pairs Around the Central Atom Linear Bent Trigonal Planar Trigonal Pyramidal Tetrahedral 2. What is the electrostatic charge of all electron groups, either shared (ie. bonds) or unshared? 3. Since all electron groups have this same charge, do they attract or repel each other? 4. How many unshared electron pairs are present in the carbon atom in CO,? 5. Considering your answers to the previous three questions, explain why CO, is not bent? 6. Explain why H,O is not linear.
ChapterU2: Smells: Molecular Structure And Properties
Section: Chapter Questions
Problem 7STP
Related questions
Question
Answer question one please and if you can answer all the other questions if you can
Transcribed Image Text:Molecular Shape:
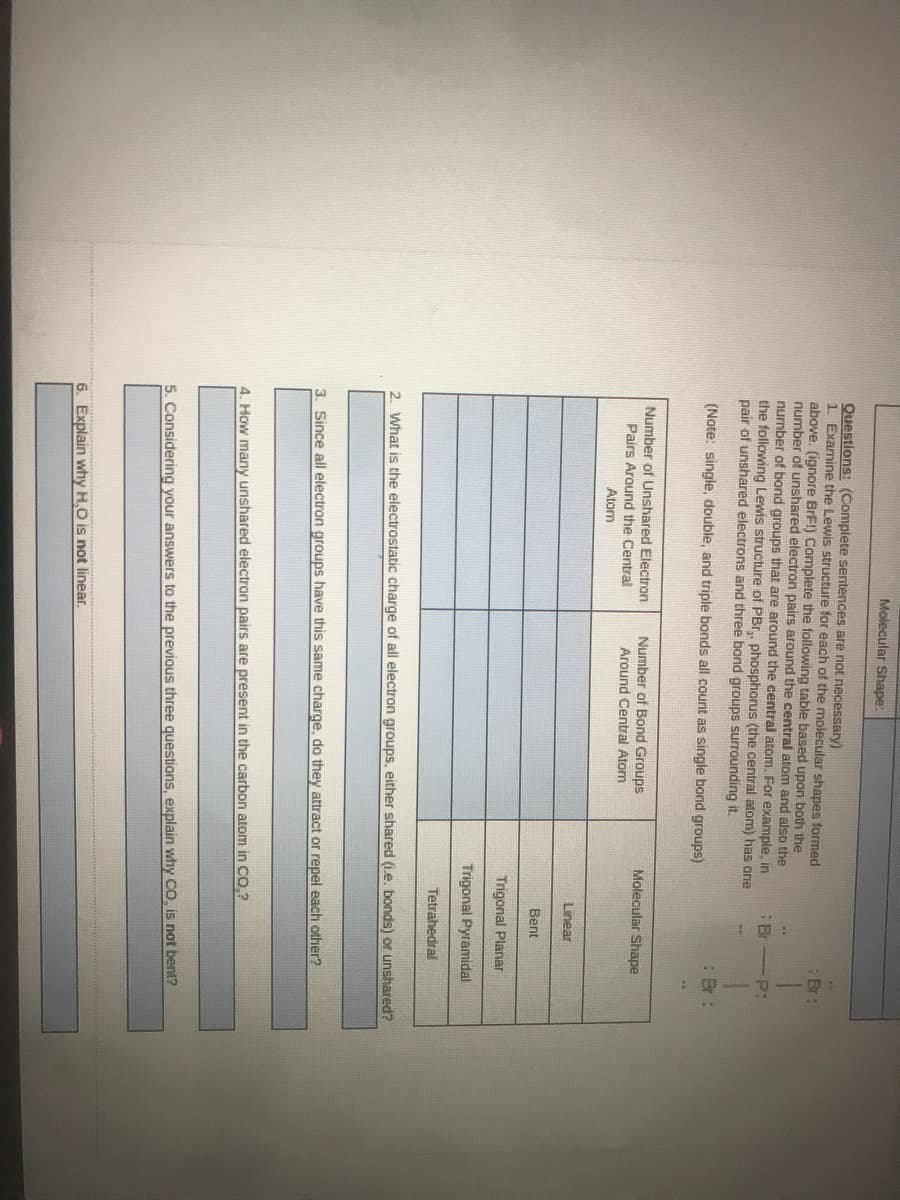
Questions: (Complete sentences are not necessary)
1 Examine the Lewis structure for each of the molecular shapes formed
above. (ignore BrF!) Complete the following table based upon both the
number of unshared electron pairs around the central atom and also the
number of band groups that are around the central atom. For example, in
the following Lewis structure of PBr,, phosphorus (the central atom) has one
pair of unshared electrons and three bond groups surrounding it.
Br
:B:
(Note: single, double, and triple bonds all count as single bond groups)
Number of Unshared Electron
Number of Bond Groups
Around Central Atom
Molecular Shape
Pairs Around the Central
Atom
Linear
Bent
Trigonal Planar
Trigonal Pyramidal
Tetrahedral
2. What is the electrostatic charge of all electron groups, either shared (Le. bonds) or unshared?
3. Since all electron groups have this same charge, do they attract or repel each other?
4. How many unshared electron pairs are present in the carbon atom in Co,?
5. Considering your answers to the previous three questions, explain why CO, is not bent?
6. Explain why H,O is not linear.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning