Directions: Use the following terms to fill-in the blanks. The terms may be used more than once. Gas Solid Liquid Phase Compress Empty space Anomaly Kinetic Energy Bonds Structure Collisions Temperature Incompressible Random Motion Absolute Zero Density Fluids Compressible 2. State the names of the phases in order of Temperature, kinetic energy, density and bond strength between atoms. In between Temperatures Lowest Temperatures Highest Temperatures Lowest Kinetic Energy In between Kinetic Energy Highest Kinetic Energy Lowest Density Highest Density High Strength Bonds Weak Bonds No Bonds 3. is directly related to temperature. As the temperature increases so does the
Directions: Use the following terms to fill-in the blanks. The terms may be used more than once. Gas Solid Liquid Phase Compress Empty space Anomaly Kinetic Energy Bonds Structure Collisions Temperature Incompressible Random Motion Absolute Zero Density Fluids Compressible 2. State the names of the phases in order of Temperature, kinetic energy, density and bond strength between atoms. In between Temperatures Lowest Temperatures Highest Temperatures Lowest Kinetic Energy In between Kinetic Energy Highest Kinetic Energy Lowest Density Highest Density High Strength Bonds Weak Bonds No Bonds 3. is directly related to temperature. As the temperature increases so does the
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter11: States Of Matter; Liquids And Solids
Section: Chapter Questions
Problem 11.157QP: Nanotechnology, or technology utilizing 1100 nm sized particles, has rapidly expanded in the past...
Related questions
Question
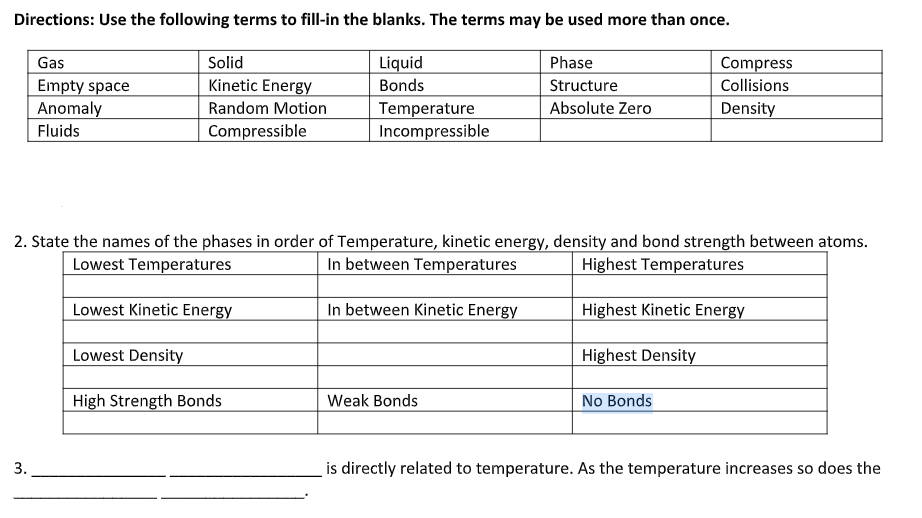
Transcribed Image Text:Directions: Use the following terms to fill-in the blanks. The terms may be used more than once.
Gas
Solid
Liquid
Phase
Compress
Empty space
Anomaly
Kinetic Energy
Bonds
Structure
Collisions
Temperature
Incompressible
Random Motion
Absolute Zero
Density
Fluids
Compressible
2. State the names of the phases in order of Temperature, kinetic energy, density and bond strength between atoms.
In between Temperatures
Lowest Temperatures
Highest Temperatures
Lowest Kinetic Energy
In between Kinetic Energy
Highest Kinetic Energy
Lowest Density
Highest Density
High Strength Bonds
Weak Bonds
No Bonds
3.
is directly related to temperature. As the temperature increases so does the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax