Does a reaction occur when aqueous solutions of chromium(II) bromide and ammonium phosphate are combined? Oyes Ono If a reaction does occur, write the net ionic equation. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank.
Does a reaction occur when aqueous solutions of chromium(II) bromide and ammonium phosphate are combined? Oyes Ono If a reaction does occur, write the net ionic equation. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 35QRT
Related questions
Question
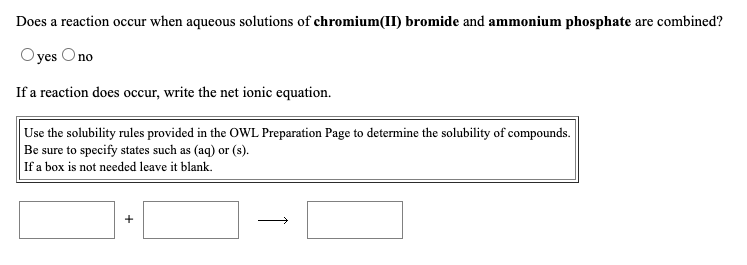
Transcribed Image Text:Does a reaction occur when aqueous solutions of chromium(II) bromide and ammonium phosphate are combined?
Oyes Ono
If a reaction does occur, write the net ionic equation.
Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.
Be sure to specify states such as (aq) or (s).
If a box is not needed leave it blank.
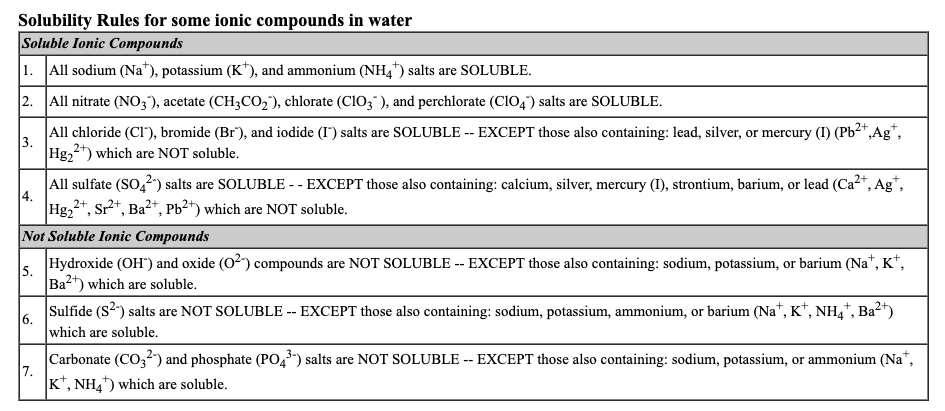
Transcribed Image Text:Solubility Rules for some ionic compounds in water
Soluble Ionic Compounds
1. All sodium (Na"), potassium (K*), and ammonium (NH,) salts are SOLUBLE.
2. All nitrate (NO3), acetate (CH3CO2), chlorate (CIO3" ), and perchlorate (ClO4) salts are SOLUBLE.
All chloride (CI"), bromide (Br"), and iodide (I) salts are SOLUBLE -- EXCEPT those also containing: lead, silver, or mercury (I) (Pb2+,Ag*,
3.
Hg,2") which are NOT soluble.
All sulfate (SO,2) salts are SOLUBLE - - EXCEPT those also containing: calcium, silver, mercury (I), strontium, barium, or lead (Ca2+, Ag*,
4.
*, Sr²*, Ba²*, Pb²+) which are NOT soluble.
Hg2
Not Soluble Ionic Compounds
Hydroxide (OH) and oxide (02) compounds are NOT SOLUBLE -- EXCEPT those also containing: sodium, potassium, or barium (Na*, K*,
5.
Ba2") which are soluble.
Sulfide (S2) salts are NOT SOLUBLE -- EXCEPT those also containing: sodium, potassium, ammonium, or barium (Na“, K*, NH,*, Ba²*)
6.
which are soluble.
Carbonate (CO,2) and phosphate (PO,) salts are NOT SOLUBLE -- EXCEPT those also containing: sodium, potassium, or ammonium (Na",
7.
K*, NH4) which are soluble.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax