$ 2,6-Di-tert-butyl-4-methylphenol, alternatively known as butylated hydroxytoluene (BHT), is used as an antioxidant in foods to"retard spoilage" ( synthesized industrially from 4-methylphenol by reaction with 2-methylpropene in the presence of phosphoric acid. Propose a mechanism for this reaction. ВНT is OH OH H&PO, 4-Methylphenol 2-Methylpropene (p-Cresol) 2,6-Di-tert-butyl-4-methylphenol "Butylated hydroxytoluene" (BHT)
$ 2,6-Di-tert-butyl-4-methylphenol, alternatively known as butylated hydroxytoluene (BHT), is used as an antioxidant in foods to"retard spoilage" ( synthesized industrially from 4-methylphenol by reaction with 2-methylpropene in the presence of phosphoric acid. Propose a mechanism for this reaction. ВНT is OH OH H&PO, 4-Methylphenol 2-Methylpropene (p-Cresol) 2,6-Di-tert-butyl-4-methylphenol "Butylated hydroxytoluene" (BHT)
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter22: Reactions Of Benzene And Its Derivatives
Section: Chapter Questions
Problem 22.23P
Related questions
Question
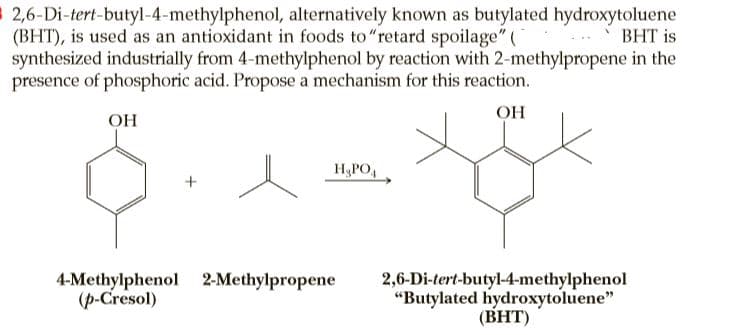
Transcribed Image Text:$ 2,6-Di-tert-butyl-4-methylphenol, alternatively known as butylated hydroxytoluene
(BHT), is used as an antioxidant in foods to"retard spoilage" (
synthesized industrially from 4-methylphenol by reaction with 2-methylpropene in the
presence of phosphoric acid. Propose a mechanism for this reaction.
ВНT is
OH
OH
H&PO,
4-Methylphenol 2-Methylpropene
(p-Cresol)
2,6-Di-tert-butyl-4-methylphenol
"Butylated hydroxytoluene"
(BHT)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 6 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
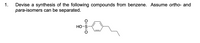
Transcribed Image Text:1.
Devise a
synthesis of the following compounds from benzene. Assume ortho- and
para-isomers can be separated.
Но
Solution
Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
