Treating cyclohexene with HBr in the presence of acetic acid gives bromocyclohexane (85%) and cyclohexyl acetate (15%). Br OCCH CH,COH + HBr Bromocyclohexane Cyclohexyl acetate (15%) Cyclohexene (85%) Propose a mechanism for the formation of the latter product.
Treating cyclohexene with HBr in the presence of acetic acid gives bromocyclohexane (85%) and cyclohexyl acetate (15%). Br OCCH CH,COH + HBr Bromocyclohexane Cyclohexyl acetate (15%) Cyclohexene (85%) Propose a mechanism for the formation of the latter product.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section: Chapter Questions
Problem 20.42P: Bicyclo-2,5-heptadiene can be prepared in two steps from cyclopentadiene and vinyl chloride. Provide...
Related questions
Question
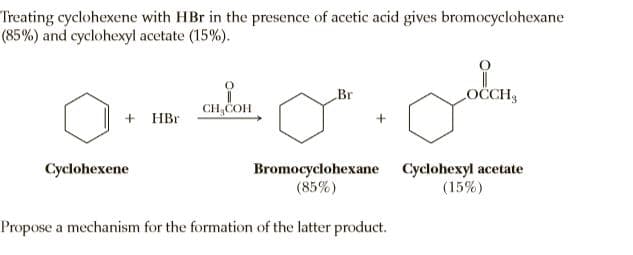
Transcribed Image Text:Treating cyclohexene with HBr in the presence of acetic acid gives bromocyclohexane
(85%) and cyclohexyl acetate (15%).
Br
OCCH
CH,COH
+ HBr
Bromocyclohexane Cyclohexyl acetate
(15%)
Cyclohexene
(85%)
Propose a mechanism for the formation of the latter product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
