Draw a full Lewis structure for each following compound. Include lone pairs and non-zero formal charges as necessary. a. HCN, hydrogen cyanide b. Si(OH)4, orthosilicic acid c. and d. are in photo attached. Look at cyclobutan-2,2-diol from d. Write the hybridizaOon of either oxygen atom (sp, sp2, or sp3). In Si(OH)4 (from question b.), write the hybridization of the silicon atom. Write compound in in this previous question that should exhibit a strong, sharp peak at 1712 cm–1 in its IR spectrum.
Draw a full Lewis structure for each following compound. Include lone pairs and non-zero formal charges as necessary. a. HCN, hydrogen cyanide b. Si(OH)4, orthosilicic acid c. and d. are in photo attached. Look at cyclobutan-2,2-diol from d. Write the hybridizaOon of either oxygen atom (sp, sp2, or sp3). In Si(OH)4 (from question b.), write the hybridization of the silicon atom. Write compound in in this previous question that should exhibit a strong, sharp peak at 1712 cm–1 in its IR spectrum.
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter28: Nitration Of Methyl Benzoate
Section: Chapter Questions
Problem 1Q
Related questions
Question
Draw a full Lewis structure for each following compound. Include lone pairs and
non-zero formal charges as necessary.
a. HCN, hydrogen cyanide
b. Si(OH)4, orthosilicic acid
c. and d. are in photo attached.
Look at cyclobutan-2,2-
oxygen atom (sp, sp2, or sp3).
In Si(OH)4 (from question b.), write the hybridization of the silicon atom.
Write compound in in this previous question that should exhibit a strong, sharp peak at 1712
cm–1 in its IR spectrum.
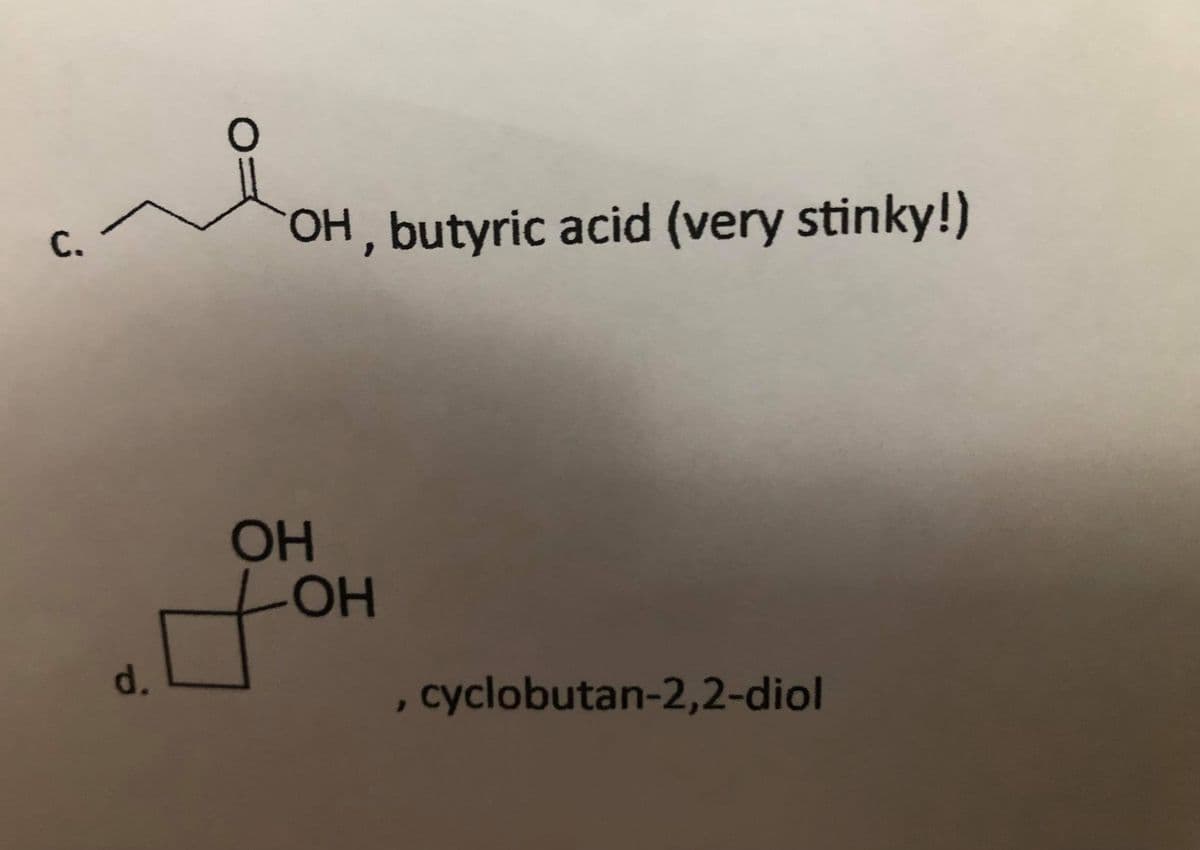
Transcribed Image Text:С.
OH, butyric acid (very stinky!)
он
LOH
HO-
d.
cyclobutan-2,2-diol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 5 images

Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning