Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 21QAP: Given the following data about xenon,...
Related questions
Question
I only need help with #2 please
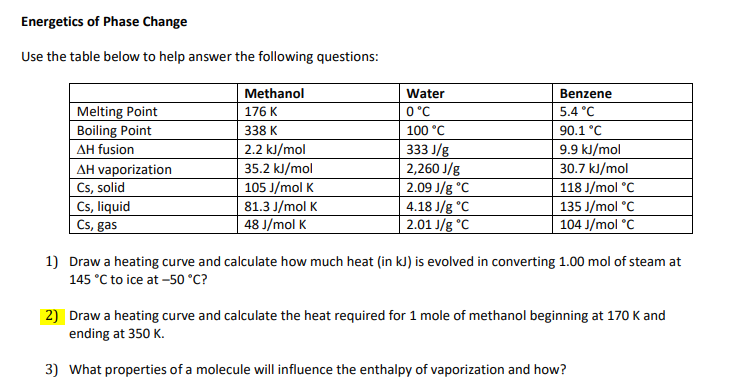
Transcribed Image Text:Energetics of Phase Change
Use the table below to help answer the following questions:
Methanol
Water
Benzene
Melting Point
Boiling Point
176 K
0°C
5.4 °C
338 K
100 °C
90.1 °C
2.2 kJ/mol
35.2 kJ/mol
105 J/mol K
81.3 J/mol K
48 J/mol K
333 J/g
2,260 J/g
2.09 J/g °C
4.18 J/g °C
2.01 J/g °C
9.9 kJ/mol
30.7 kJ/mol
118 J/mol °C
135 J/mol °C
104 J/mol °C
AH fusion
AH vaporization
Cs, solid
Cs, liquid
Cs, gas
1) Draw a heating curve and calculate how much heat (in kJ) is evolved in converting 1.00 mol of steam at
145 °C to ice at -50 °C?
|2) Draw a heating curve and calculate the heat required for 1 mole of methanol beginning at 170 K and
ending at 350 K.
3) What properties of a molecule will influence the enthalpy of vaporization and how?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,