Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter13: The Geometrical Structure Of Molecules-an Experiment Using Molecular Models
Section: Chapter Questions
Problem 4ASA: a. How many sticks did you need to make the skeleton structure?____________ b. How many sticks are...
Related questions
Question
draw a resonance structure
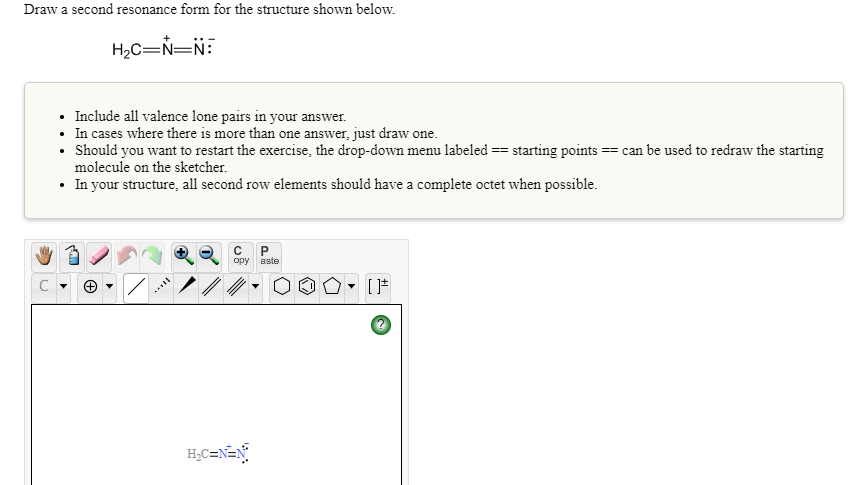
Transcribed Image Text:Draw a second resonance form for the structure shown below.
H2C=N=N:
• Include all valence lone pairs in your answer.
• In cases where there is more than one answer, just draw one.
• Should you want to restart the exercise, the drop-down menu labeled == starting points == can be used to redraw the starting
molecule on the sketcher.
• In your structure, all second row elements should have a complete octet when possible.
opy
aste
ノ/
H;C=N=N°
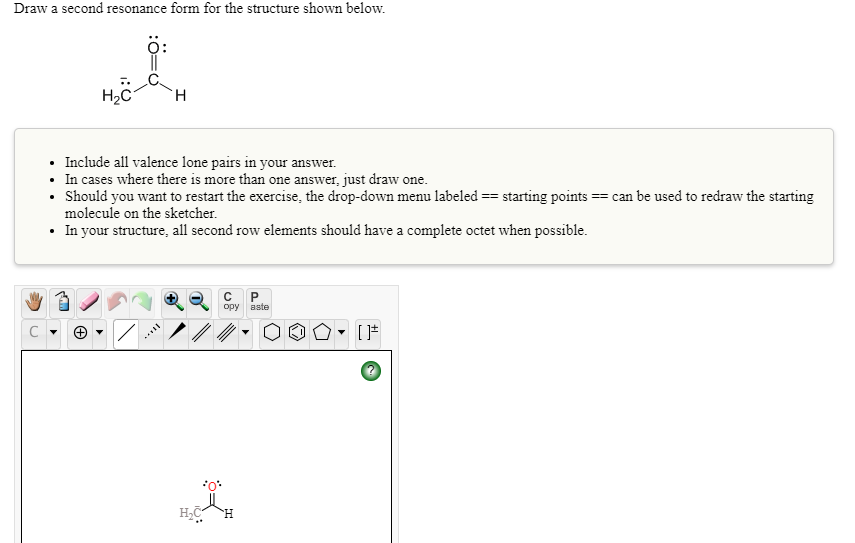
Transcribed Image Text:Draw a second resonance form for the structure shown below.
Include all valence lone pairs in your answer.
• In cases where there is more than one answer, just draw one.
Should you want to restart the exercise, the drop-down menu labeled == starting points == can be used to redraw the starting
molecule on the sketcher.
• In your structure, all second row elements should have a complete octet when possible.
opy
aste
H.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning