Draw the decay diagram for the decay of 32 Si to 325. If the total energy of decay for the beta decay of 32Si to 32P is 0.2243 MeV, and that for 32P to 32S is 1.7105 MeV, and the mass of PS is 31.972071 u, calculate the atomic mass of 32Si. The problem doesn't ask, but the two decays can be represented as Si - P + -je P → S+ -je
Draw the decay diagram for the decay of 32 Si to 325. If the total energy of decay for the beta decay of 32Si to 32P is 0.2243 MeV, and that for 32P to 32S is 1.7105 MeV, and the mass of PS is 31.972071 u, calculate the atomic mass of 32Si. The problem doesn't ask, but the two decays can be represented as Si - P + -je P → S+ -je
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter19: Radioactivity And Nuclear Energy
Section: Chapter Questions
Problem 89AP
Related questions
Question
100%

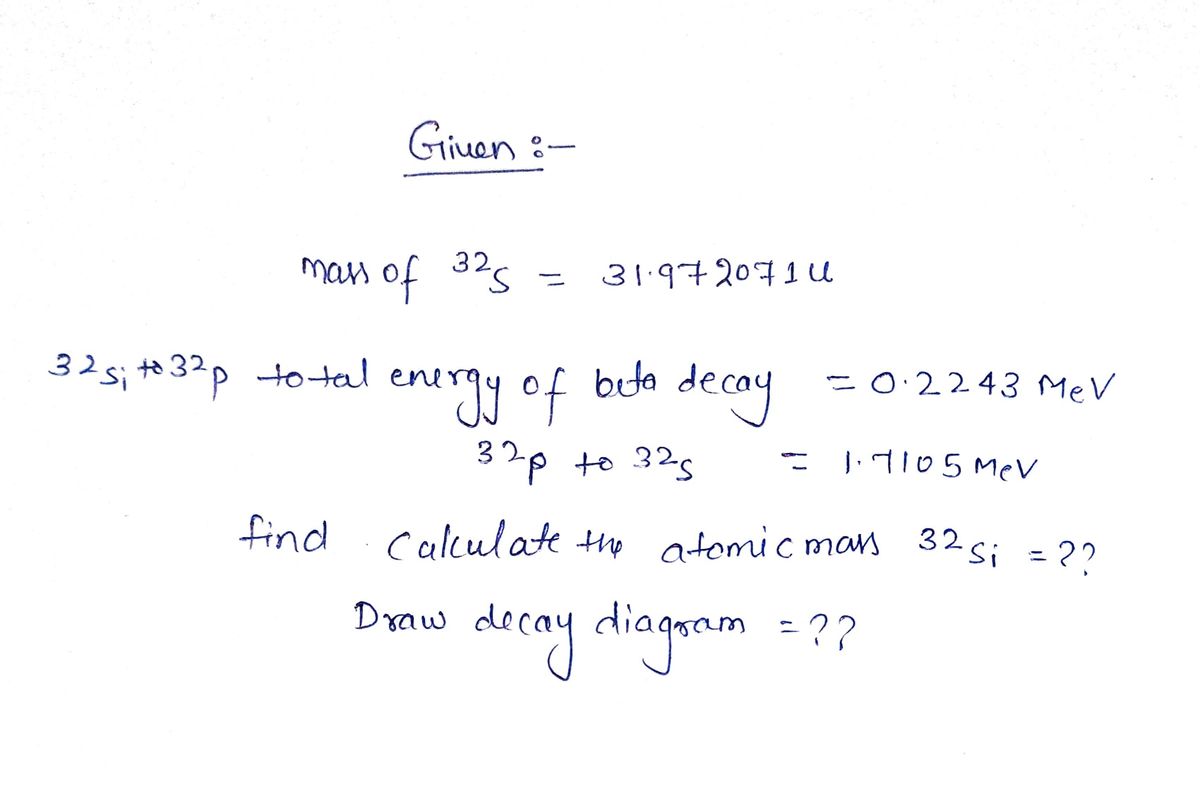
Transcribed Image Text:Draw the decay diagram for the decay of 32Si to 32S. If the total energy of decay for
the beta decay of 32Si to 32P is 0.2243 MeV, and that for 32P to 32S is 1.7105 MeV,
and the mass of 2S is 31.972071 u, calculate the atomic mass of 32Si.
The problem doesn't ask, but the two decays can be represented as
Si – P + -je
P → S + -1€
Expert Solution
Step 1
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning