Draw the 'H NMR spectrum for the compound shown below. The expected chemical shift (8, ppm) and splitting pattern (multiplicity) for each peak should be illustrated. OCH2CH3 OCH2CH3
Draw the 'H NMR spectrum for the compound shown below. The expected chemical shift (8, ppm) and splitting pattern (multiplicity) for each peak should be illustrated. OCH2CH3 OCH2CH3
Chapter30: Orbitals And Organic Chemistry: Pericyclic Reactions
Section30.SE: Something Extra
Problem 37AP: The 1H NMR spectrum of bullvalene at 100 C consists only of a single peak at 4.22 . Explain.
Related questions
Question
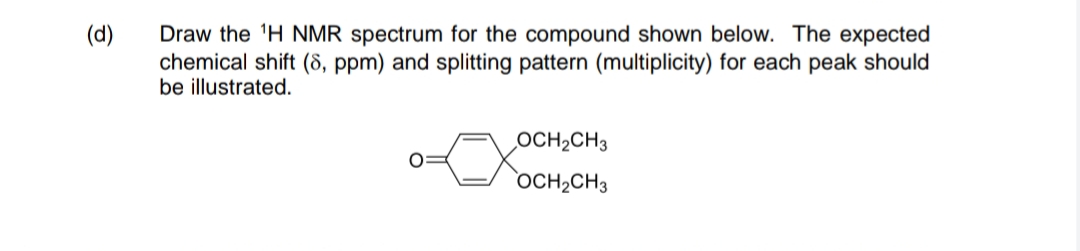
Transcribed Image Text:Draw the 'H NMR spectrum for the compound shown below. The expected
chemical shift (8, ppm) and splitting pattern (multiplicity) for each peak should
be illustrated.
(d)
OCH2CH3
OCH2CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole