Professor Modyn owns another piston-cylinder device, which initially (State 1) contains 0.236 m³ of saturated water vapor at 375.0 kPa. Heat is now slowly transferred to the steam until the volume doubles. Consider the expansion a two-step process. First, constant volume heating occurs until the pressure reaches 500.0 kPa (State 2). Finally, a constant pressure expansion occurs, resulting in the doubling of the initial volume (State 3). Note: The IUPAC sign convention for work is used. Work into the system has a positive value. What is the final temperature (State 3)? 832.02 final temperature: Incorrect What is the overall work interaction term, W13? work: -118 kJ What is the overall heat interaction term, Q13? 168.939 heat: kJ Incorrect
Professor Modyn owns another piston-cylinder device, which initially (State 1) contains 0.236 m³ of saturated water vapor at 375.0 kPa. Heat is now slowly transferred to the steam until the volume doubles. Consider the expansion a two-step process. First, constant volume heating occurs until the pressure reaches 500.0 kPa (State 2). Finally, a constant pressure expansion occurs, resulting in the doubling of the initial volume (State 3). Note: The IUPAC sign convention for work is used. Work into the system has a positive value. What is the final temperature (State 3)? 832.02 final temperature: Incorrect What is the overall work interaction term, W13? work: -118 kJ What is the overall heat interaction term, Q13? 168.939 heat: kJ Incorrect
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 81AP
Related questions
Question
Since this is regarding water we can't use the
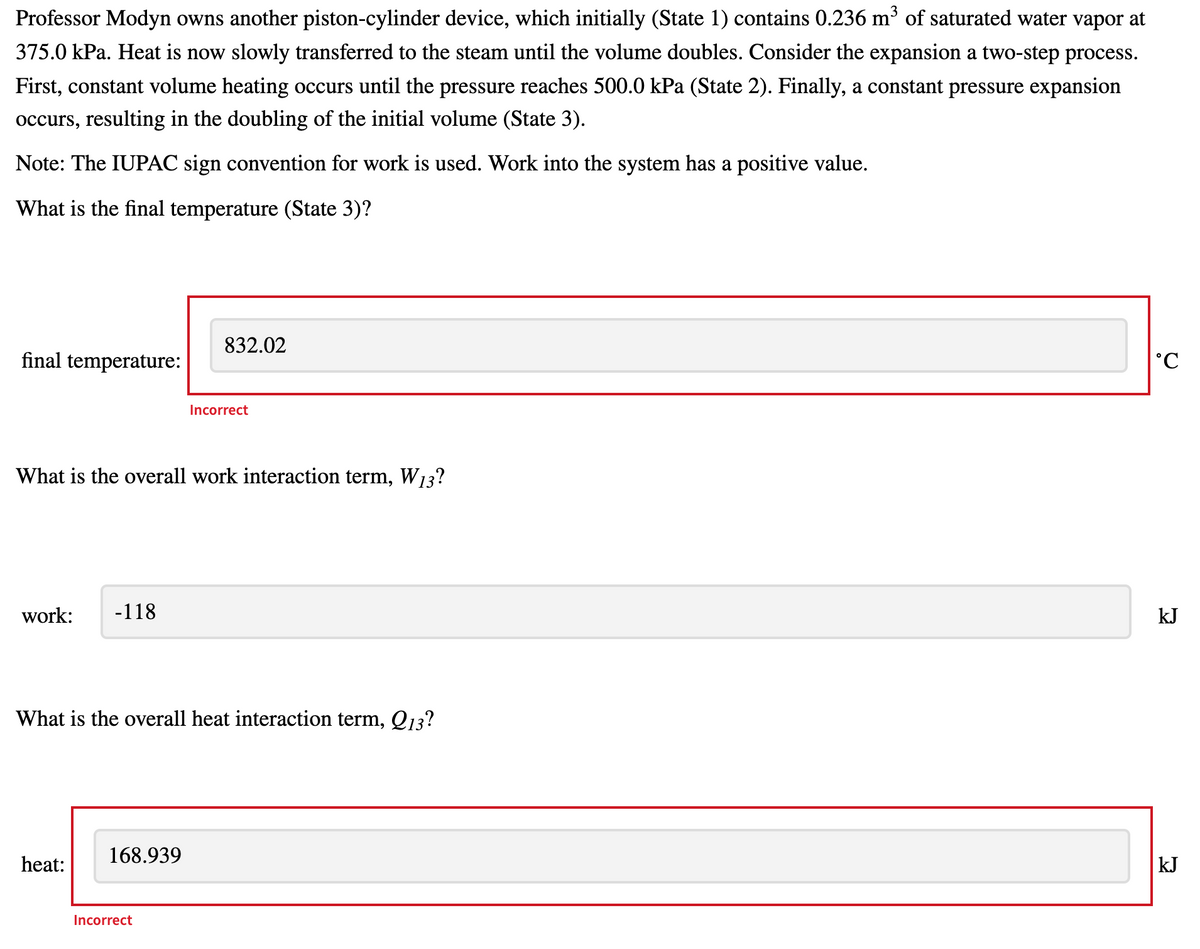
Transcribed Image Text:Professor Modyn owns another piston-cylinder device, which initially (State 1) contains 0.236 m³ of saturated water vapor at
375.0 kPa. Heat is now slowly transferred to the steam until the volume doubles. Consider the expansion a two-step process.
First, constant volume heating occurs until the pressure reaches 500.0 kPa (State 2). Finally, a constant pressure expansion
occurs, resulting in the doubling of the initial volume (State 3).
Note: The IUPAC sign convention for work is used. Work into the system has a positive value.
What is the final temperature (State 3)?
832.02
final temperature:
ɔ.
Incorrect
What is the overall work interaction term, W13?
work:
-118
kJ
What is the overall heat interaction term, Q13?
168.939
heat:
kJ
Incorrect
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning