Draw the Lewis structure of CH₂NH and then choose the appropriate pair of hybridization states for the two central atoms. Your answer choice is independent of the orientation of your drawn structure.
Draw the Lewis structure of CH₂NH and then choose the appropriate pair of hybridization states for the two central atoms. Your answer choice is independent of the orientation of your drawn structure.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter5: Resonance
Section: Chapter Questions
Problem 18CTQ: Consider the polarization of the C=O bond in the molecule acetone a. (E) Which atom (C or O) is...
Related questions
Question
Draw the Lewis structure of CH₂NH and then choose the appropriate pair of hybridization states for the two central atoms. Your answer choice is independent of the orientation of your drawn structure.
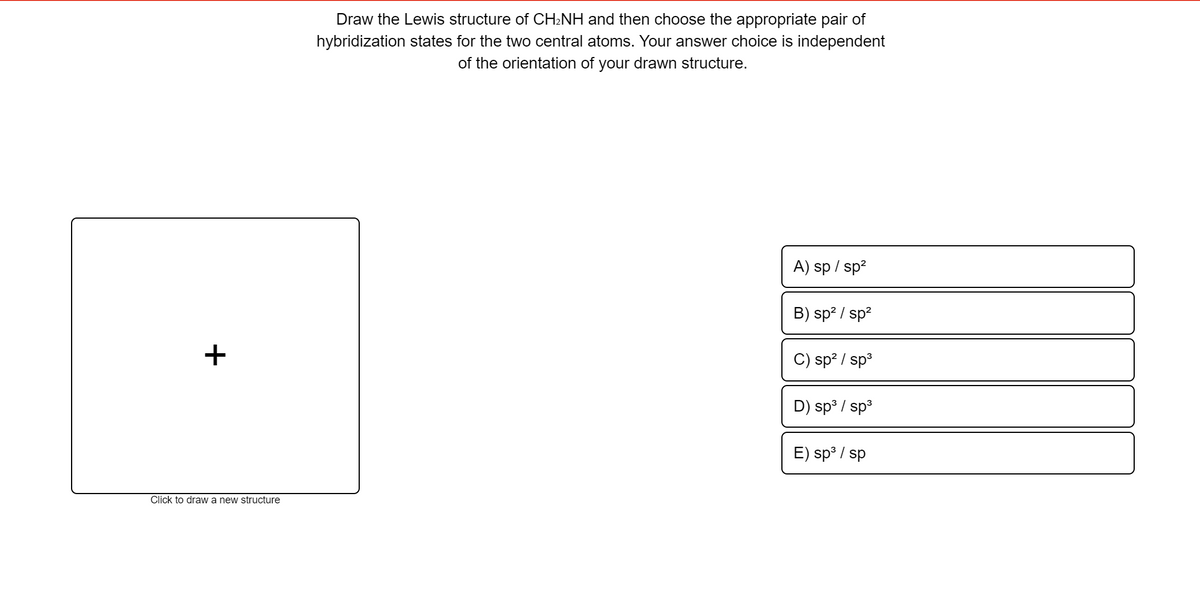
Transcribed Image Text:Draw the Lewis structure of CH:NH and then choose the appropriate pair of
hybridization states for the two central atoms. Your answer choice is independent
of the orientation of your drawn structure.
A) sp / sp?
B) sp? / sp?
+
C) sp? / sp3
D) sp³ / sp3
E) sp° / sp
Click to draw a new structure
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning