Draw the Lewis structure of N₂O₄ and then choose the appropriate pair of hybridization states for the two central atoms. Your answer choice is independent of the orientation of your drawn structure.
Draw the Lewis structure of N₂O₄ and then choose the appropriate pair of hybridization states for the two central atoms. Your answer choice is independent of the orientation of your drawn structure.
Chapter3: Mechanisms
Section: Chapter Questions
Problem 105EQ
Related questions
Question
Draw the Lewis structure of N₂O₄ and then choose the appropriate pair of hybridization states for the two central atoms. Your answer choice is independent of the orientation of your drawn structure.
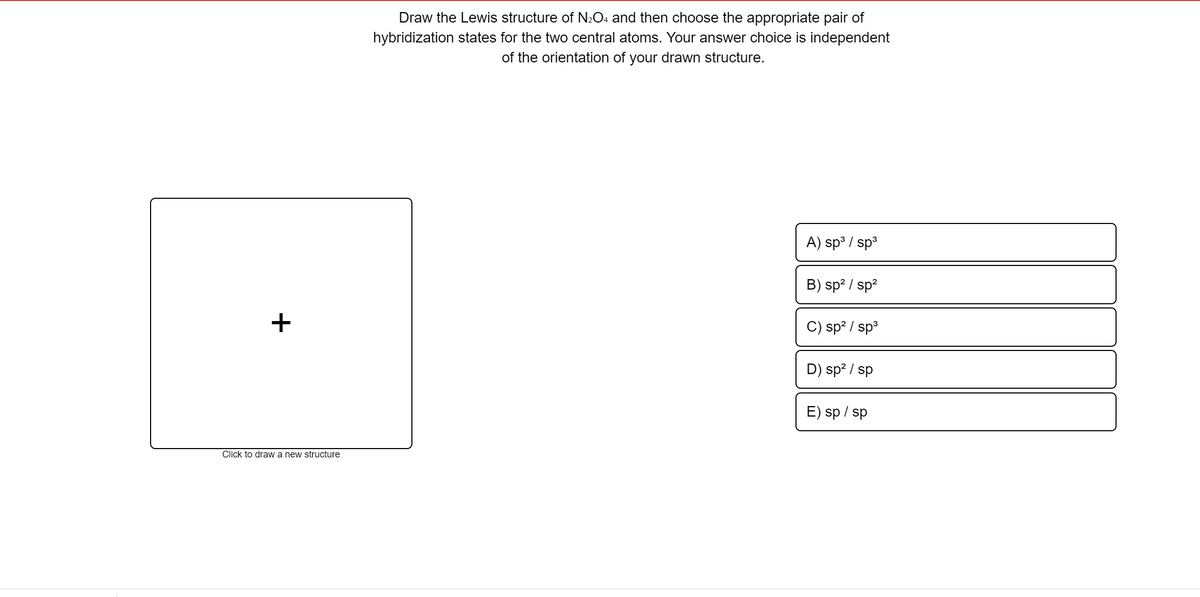
Transcribed Image Text:Draw the Lewis structure of N2O4 and then choose the appropriate pair of
hybridization states for the two central atoms. Your answer choice is independent
of the orientation of your drawn structure.
A) sp / sp3
B) sp? / sp?
+
C) sp? / sp3
D) sp? / sp
E) sp / sp
Click to draw a new structure
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning