Draw the Lewis structures and predict the hybridization (sp, sp2, sp3), geometry (linear, trigonal, tetrahedral) and bond angles (180°, 120°, 109.5°) for the central atoms in the following compounds. a. Cin CH3CCH b. C & O in CH3OCH3 c. C & N in CH3CHNCH3
Draw the Lewis structures and predict the hybridization (sp, sp2, sp3), geometry (linear, trigonal, tetrahedral) and bond angles (180°, 120°, 109.5°) for the central atoms in the following compounds. a. Cin CH3CCH b. C & O in CH3OCH3 c. C & N in CH3CHNCH3
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter3: Electron Orbitals
Section: Chapter Questions
Problem 8E
Related questions
Question
Kindly answer the following questions from 1 to 3. As per your guidelines, only solve the first three questions.
1. Draw the Lewis structures and predict the hybridization (sp, sp2, sp3), geometry (linear, trigonal, tetrahedral) and bond angles (180°, 120°, 109.5°) for the central atoms in the following compounds.
2. Give the systematic (IUPAC) name for each of the following
3. Given the IUPAC name, draw the structure of the following compounds.
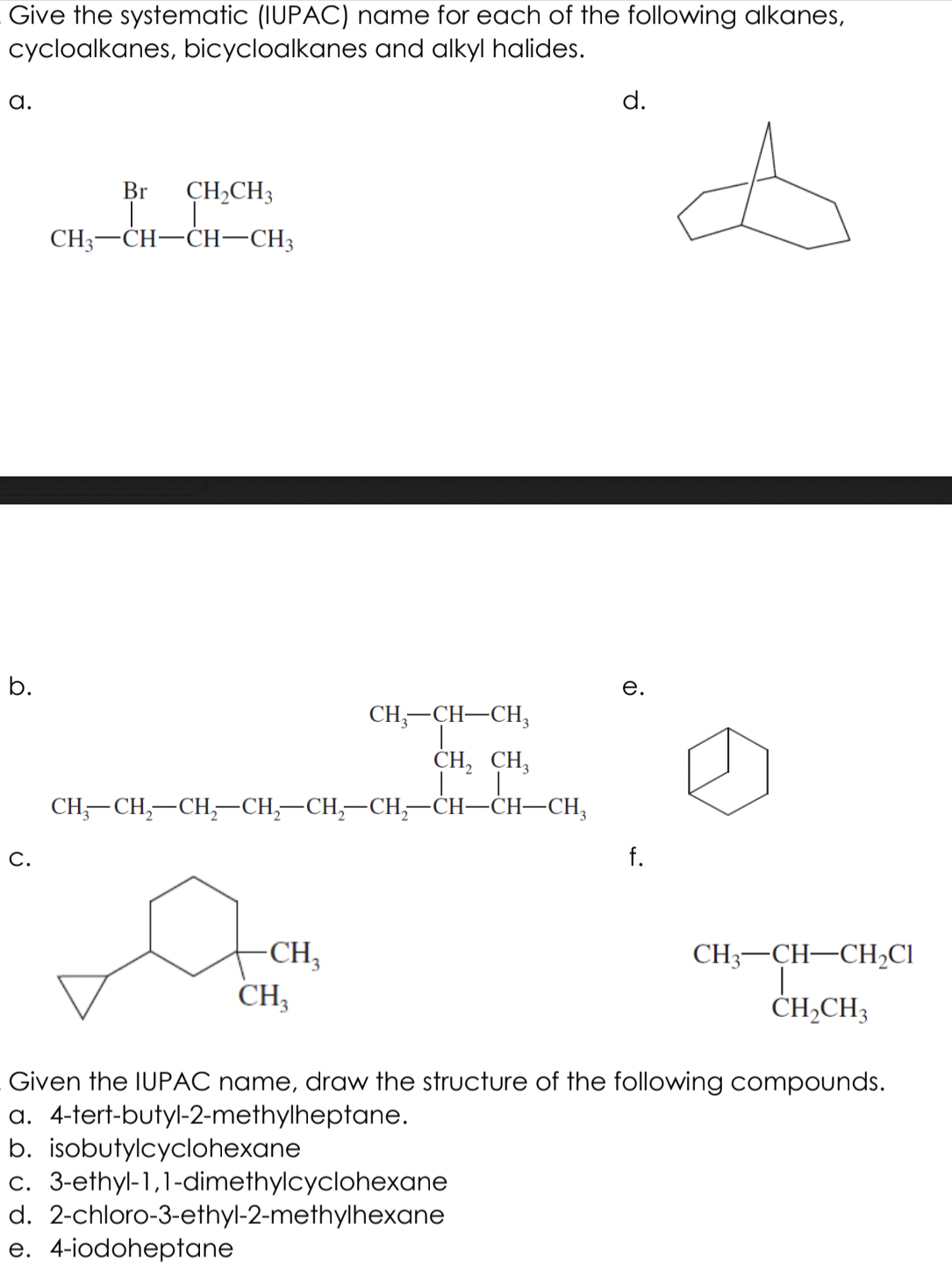
Transcribed Image Text:Give the systematic (IUPAC) name for each of the following alkanes,
cycloalkanes, bicycloalkanes and alkyl halides.
а.
d.
Br
CH,CH3
CH3-CH-CH-CH3
b.
е.
CH;-CH-CH,
CH, CH,
CH,—CH,—СH,СH, —CH, —СH;—сH—CH—СH,
C.
f.
-CH,
CH3
CH3-CH-CH2CI
CH,CH3
Given the IUPAC name, draw the structure of the following compounds.
a. 4-tert-butyl-2-methylheptane.
b. isobutylcyclohexane
c. 3-ethyl-1,1-dimethylcyclohexane
d. 2-chloro-3-ethyl-2-methylhexane
e. 4-iodoheptane

Transcribed Image Text:Draw the Lewis structures and predict the hybridization (sp, sp2, sp3), geometry
(linear, trigonal, tetrahedral) and bond angles (180°, 120°, 109.5°) for the central
atoms in the following compounds.
a. Cin CH3CCH
b. C & O in CH3OCH3
c. C & N in CH3CHNCH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning