Draw the molecular orbital energy diagram (like we did in class, not the fancy general chemistry diagrams) for the bond between the nitrogen atom labeled a and the carbon atom labeled b in the following molecule. Clearly indicate which atomic orbitals are interacting, which resulting orbital the electrons are in, and label HOMO, LUMO, bonding and antibonding orbitals, and sigma (o), sigma* (o*), pi (n), and pi* (7*) where appropriate.
Draw the molecular orbital energy diagram (like we did in class, not the fancy general chemistry diagrams) for the bond between the nitrogen atom labeled a and the carbon atom labeled b in the following molecule. Clearly indicate which atomic orbitals are interacting, which resulting orbital the electrons are in, and label HOMO, LUMO, bonding and antibonding orbitals, and sigma (o), sigma* (o*), pi (n), and pi* (7*) where appropriate.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter4: Nomenclature
Section: Chapter Questions
Problem 25A
Related questions
Question
Draw the molecular orbital energy diagram (like we did in class, not the fancy general chemistry diagrams) for the bond between the nitrogen atom labeled a and the carbon atom labeled b in the following molecule. Clearly indicate which atomic orbitals are interacting, which resulting orbital the electrons are in, and label HOMO, LUMO, bonding and antibonding orbitals, and sigma (o), sigma* (o*), pi (n), and pi* (7*) where appropriate.
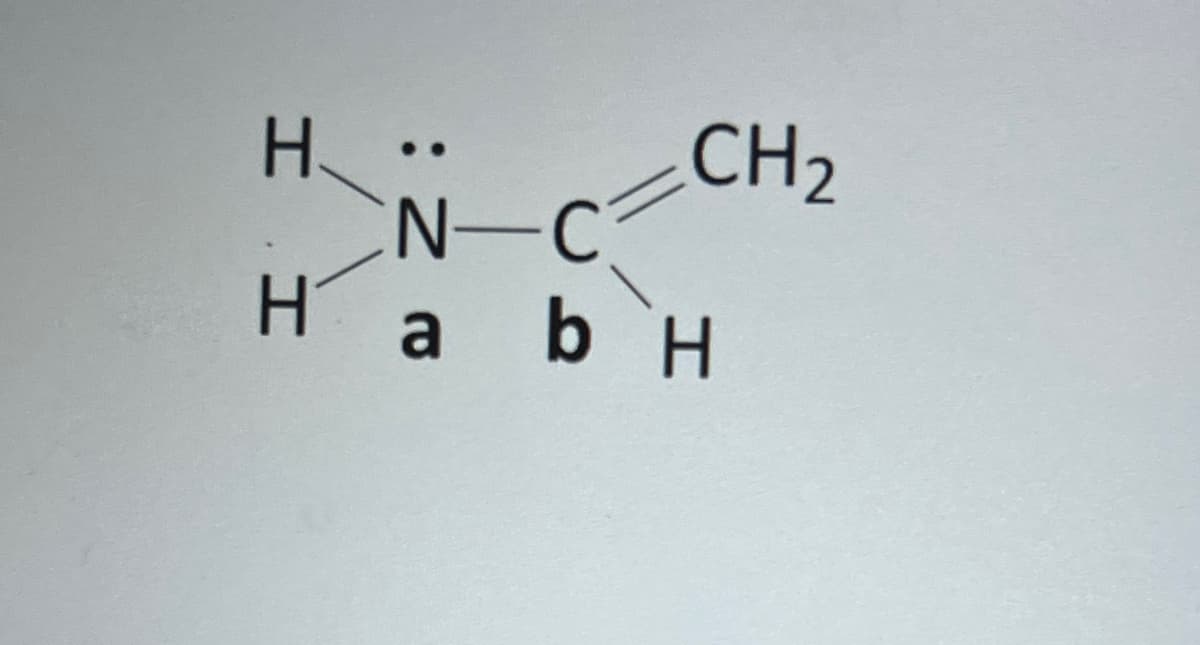
Transcribed Image Text:І І
Н.
CH₂
N-C
На ьн
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning