Dwayne Theoretical yield of chemical reactions Gaseous ethane (CH,CH,) reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). what is the theoretical yield of water formed from the reaction of 4.21 g of ethane and 20.5 g of oxygen gas? Round your answer to 3 significant figures.
Dwayne Theoretical yield of chemical reactions Gaseous ethane (CH,CH,) reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). what is the theoretical yield of water formed from the reaction of 4.21 g of ethane and 20.5 g of oxygen gas? Round your answer to 3 significant figures.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter4: Stoichiometry Of Chemical Reactions
Section: Chapter Questions
Problem 67E: Toluene, C6H5CH3, is oxidized by air under carefully controlled conditions to benzoic acid,...
Related questions
Question
100%
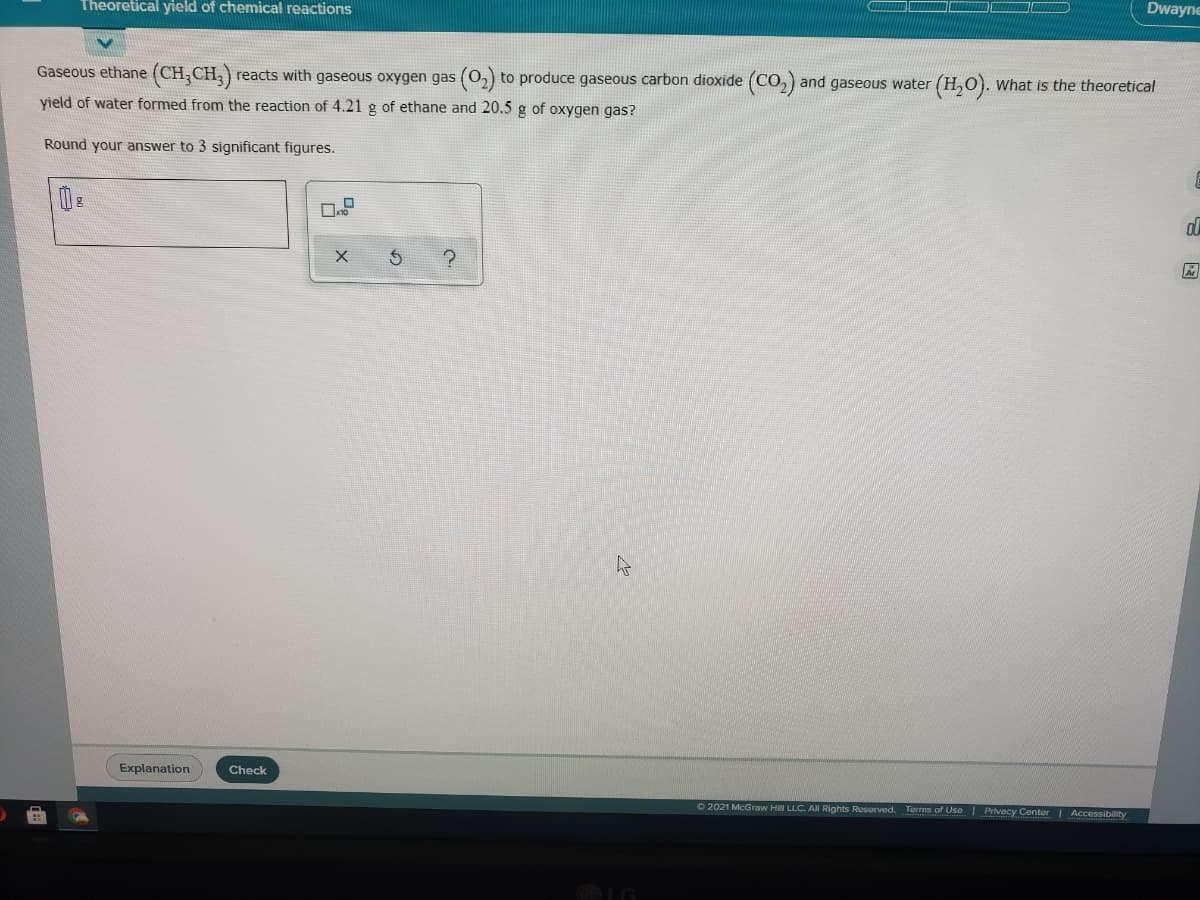
Transcribed Image Text:Dwayne
Theoretical yield of chemical reactions
Gaseous ethane (CH;CH;)
reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). what is the theoretical
yield of water formed from the reaction of 4.21 g of ethane and 20.5 g of oxygen gas?
Round your answer to 3 significant figures.
Explanation
Check
O 2021 McGraw Hill LLC. AIl Rights Reserved. Terms of Use Privacy Center Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning