e) Note: d-orbitals are not important for the bonding description of CIF5. Give a brief reason why. ) Which molecular orbital do you predict to be the most bonding? Draw a picture of this orbital including the phase. g) In part c, you found the symmetry of some d orbitals on the central Cl atom. Computational and experimental work confirm that the d-orbitals are not important for the bonding description of CIF5 or other main group molecules with so-called "expanded octets." Think about the requirements for significant interaction we discussed in class and give a brief reason why d-orbital involvement is unlikely. (Hint: You should be able to offer a reasonable explanation without looking up any numbers or doing any calculations.)
e) Note: d-orbitals are not important for the bonding description of CIF5. Give a brief reason why. ) Which molecular orbital do you predict to be the most bonding? Draw a picture of this orbital including the phase. g) In part c, you found the symmetry of some d orbitals on the central Cl atom. Computational and experimental work confirm that the d-orbitals are not important for the bonding description of CIF5 or other main group molecules with so-called "expanded octets." Think about the requirements for significant interaction we discussed in class and give a brief reason why d-orbital involvement is unlikely. (Hint: You should be able to offer a reasonable explanation without looking up any numbers or doing any calculations.)
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter13: Introduction To Symmetry In Quantum Mechanics
Section: Chapter Questions
Problem 13.32E
Related questions
Question
Help me please
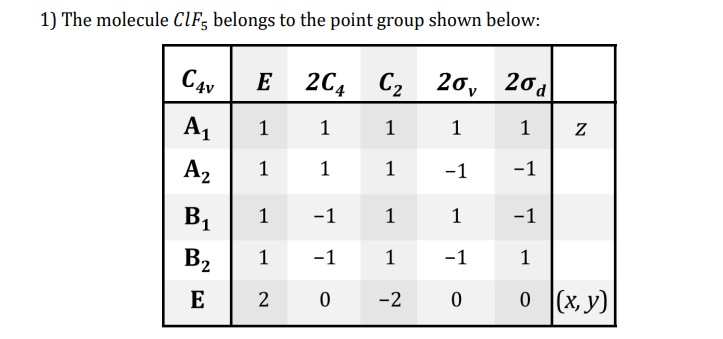
Transcribed Image Text:1) The molecule CIF; belongs to the point group shown below:
C4v
E
2C4
C2 20, 2o
20a
A1
1
1
1
1
A2
1
1
-1
-1
B1
1
-1
1
1
-1
1
B2
-1
1
-1
1
2 0
0 (x, y)
E
-2

Transcribed Image Text:e) Note: d-orbitals are not important for the bonding description of CIF5. Give a
brief reason why.
f) Which molecular orbital do you predict to be the most bonding? Draw a
picture of this orbital including the phase.
g) In part c, you found the symmetry of some d orbitals on the central Cl atom.
Computational and experimental work confirm that the d-orbitals are not
important for the bonding description of ClF; or other main group molecules
with so-called "expanded octets." Think about the requirements for significant
interaction we discussed in class and give a brief reason why d-orbital
involvement is unlikely. (Hint: You should be able to offer a reasonable
explanation without looking up any numbers or doing any calculations.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,